Solved 14. The latent heat of vaporization of isopropyl

By A Mystery Man Writer
Answer to Solved 14. The latent heat of vaporization of isopropyl

Materials Proceedings, Free Full-Text

Last lesson?. Thermal capacity Thermal capacity is the amount of energy needed to raise the temperature of a substance by 1K. - ppt download

Determination of ethanol, isopropyl alcohol and methanol in alcohol-based hand sanitiser to ensure product quality, safety and efficacy
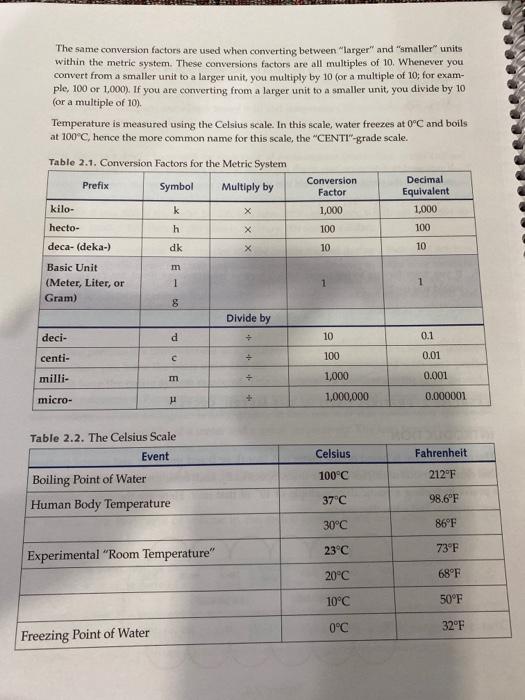
SOLVED: Part 3: Latent Heat of Vaporization of Water Data: Mass of water initially: 5.8 g Mass of aluminum cup: 543.3 g Initial water-Al temperature: Twi Mass of water vapor added: 26.8
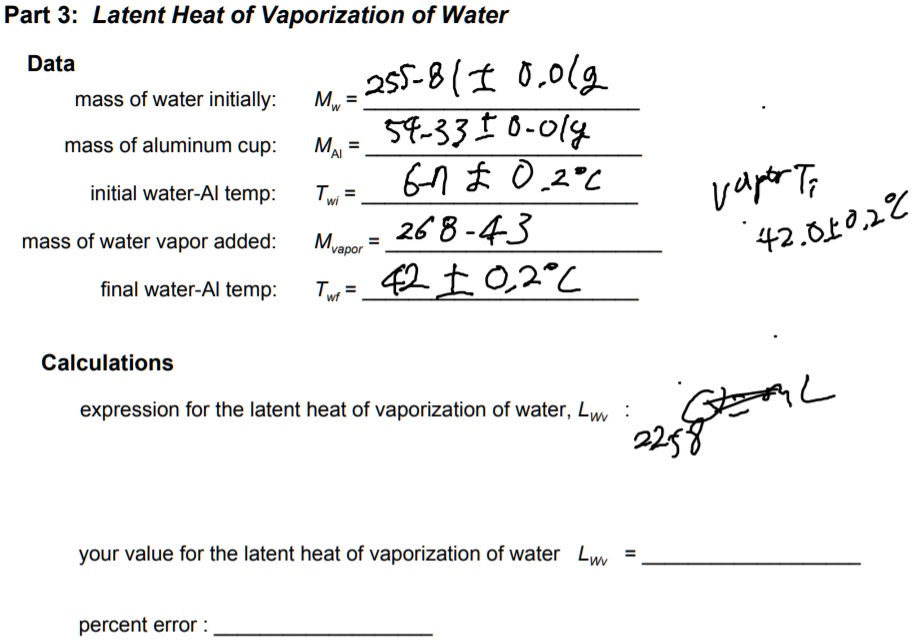
Solved Suppose that 1.05 g of rubbing alcohol (C3H8O)

Supply for Lab Chemical Laboratory Chemical Specific Reagents Edible Alcohol Disinfectants Isopropyl 99.9% Absolute Ethanol - China Ethanol Absolute, Absolute Ethanol

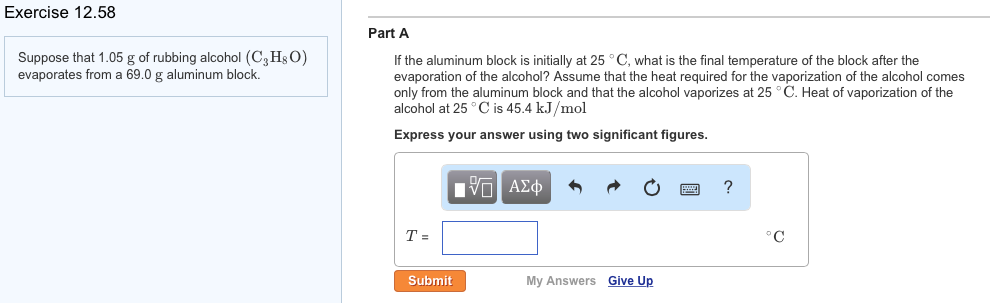
SOLVED: Isopropyl alcohol has a heat of vaporization of 3.99 * 10^3 mol^-1 and a boiling point of 82.30 °C at 1.000 atm. Using the Clausius-Clapeyron equation, calculate the vapor pressure of
SOLVED: Isopropyl alcohol has a heat of vaporization of 3.99 * 10^3 mol^-1 and a boiling point of 82.30 °C at 1.000 atm. Using the Clausius-Clapeyron equation, calculate the vapor pressure of

Polymers, Free Full-Text
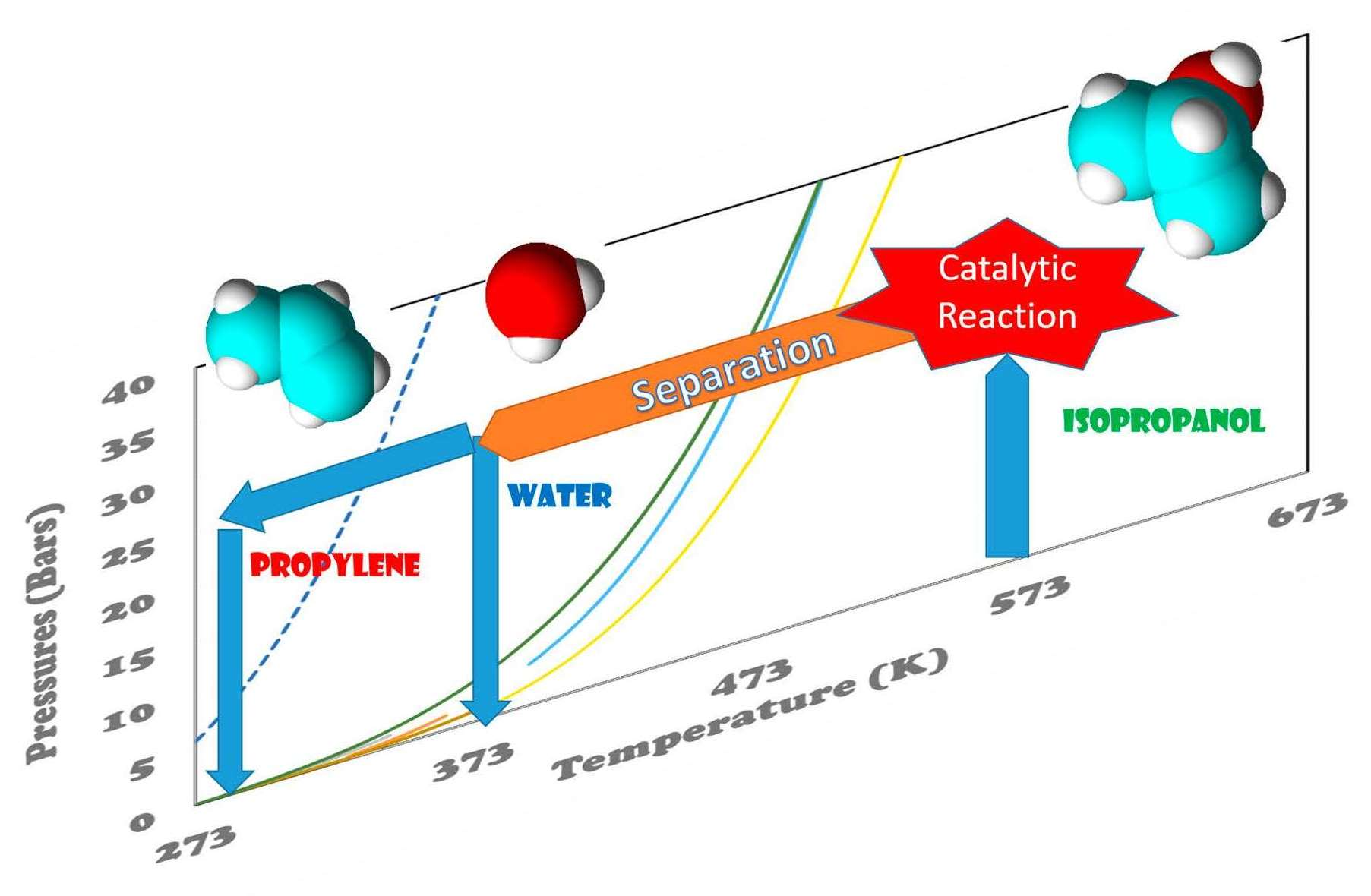
Catalysts, Free Full-Text
- How to Convert Fahrenheit to Celsius

- Solved The same conversion factors are used when converting
- Dromkeen Rhodesian Ridgebacks - Celsius to Fahrenheit conversion 32C=89F, 28C=82F, 24C=75F, 20C=68F 16C=60F, 12C=53F
- Converting Temperature with Answer Key
- [PRODUCTION 2ND] Convert - Draft Collar - 950 -10°F (-23°C) - Reg/XWide - Navy 10D/Salmon Orange 7D (218795.001)

- Aim'n Navy Stripe Tights L Leggings are not pants, Striped tights, Pants for women

- Turn Heads and Hearts this Durga Puja with our Exquisite Ethnic Collection! 🤩 From Bodhon to Bishorjon, be festive-ready with Go Colo
- camouflage pants outfit heels|TikTok Search

- 8 Best Swim Diapers

- Girls Novelty Lined Camisole - City Threads USA