200 g of a sample of limestone liberates 66 g of CO2 on heating
By A Mystery Man Writer
200 g of a sample of limestone liberates 66 g of CO2 on heating. The percentage purity of CaCO3 in the limestone is Options:a 95
200 g of a sample of limestone liberates 66 g of CO2 on heating- The percentage purity of CaCO3 in the limestone is Options-a- 95

CHEMICAL REACTION AND EQUATIONS

When 200 g of lime strongly heated , it undergoes thermal decomposition to form 112 g of lime and

PDF) Quimica Analitica Hamilton
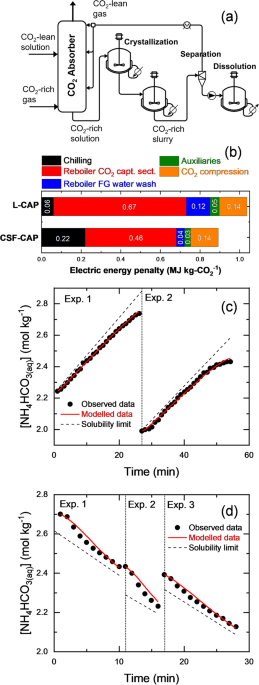
A review on chemical precipitation in carbon capture, utilization and storage, Sustainable Environment Research
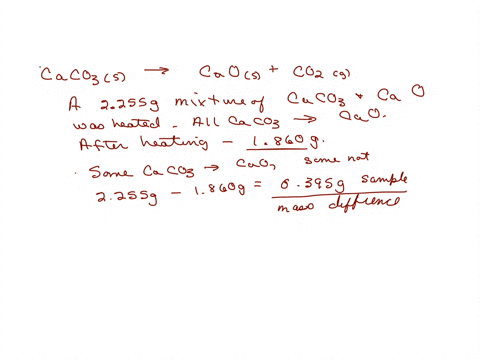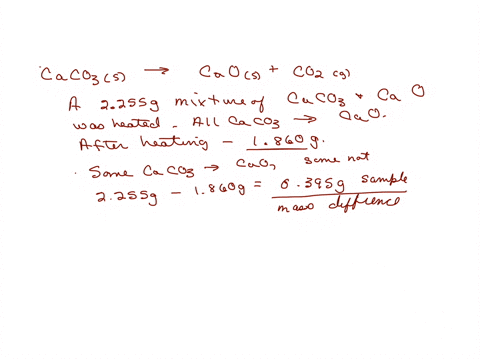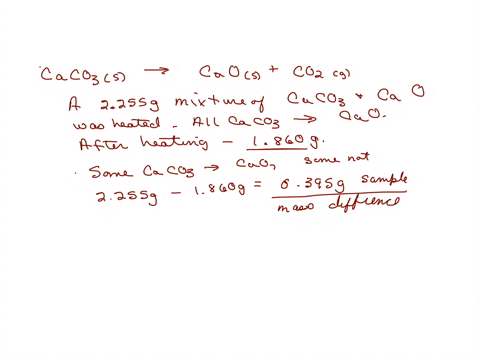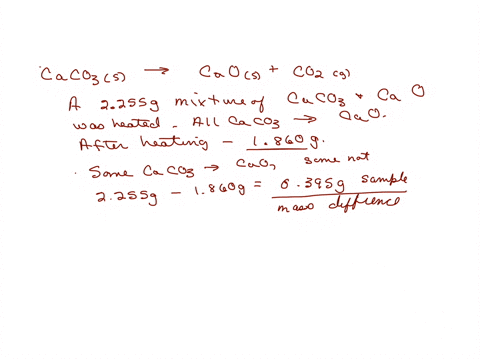
SOLVED: A sample of limestone and other soil materials was heated
PDF) A comparative study of CO2 sorption properties for different

Flame curtain pyrolysis of oil palm fronds for potential acidic soil amelioration and climate change mitigation - ScienceDirect

Combustion analysis of 0.1500 g of methyl tert-butyl ether, an o

A review on chemical precipitation in carbon capture, utilization and storage, Sustainable Environment Research

Bansal classes chemistry study material for iit jee by S.Dharmaraj

4) 15 g 8. 50 g of a sample of limestone (CaCO3) on complete decomposition gives 20 g of CO2. The percentage purity of CaCO3 in limestone is (Atomic mass of Ca =

PhEd-Some Basic Concepts of Chemistry-W.S, PDF, Mole (Unit)
- Sexy Plus Size Body Harness Bra Garter With Strappy Tops And

- Can I wear brown shoes with black pants? Blue shirt black pants, Black pants brown shoes, Blue shirt outfits

- Hugs Kisses and a lot of Birthday Wishes Women's Tank Top by Jacob Zelazny - Pixels

- Nicx
- 🥀Vintage Flax by Jeanne Engelhart Rust Red 100% - Depop

:quality(80)/anatofee/catalog/7683-4144-g.jpg)


)
