FDA Cleared vs Approved vs Granted for Medical Devices
By A Mystery Man Writer
Ever wonder what FDA cleared vs approved vs granted actually mean? Learn the subtle yet important differences between these regulatory terms.

Emergency medical device registration procedure in China On the basis

Medical Device Databases
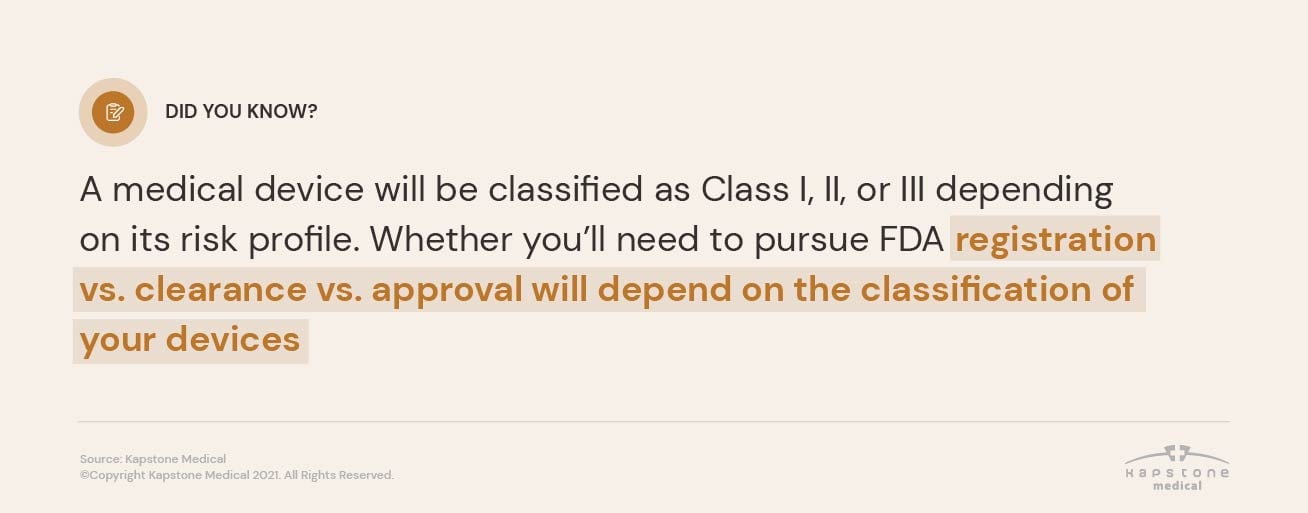
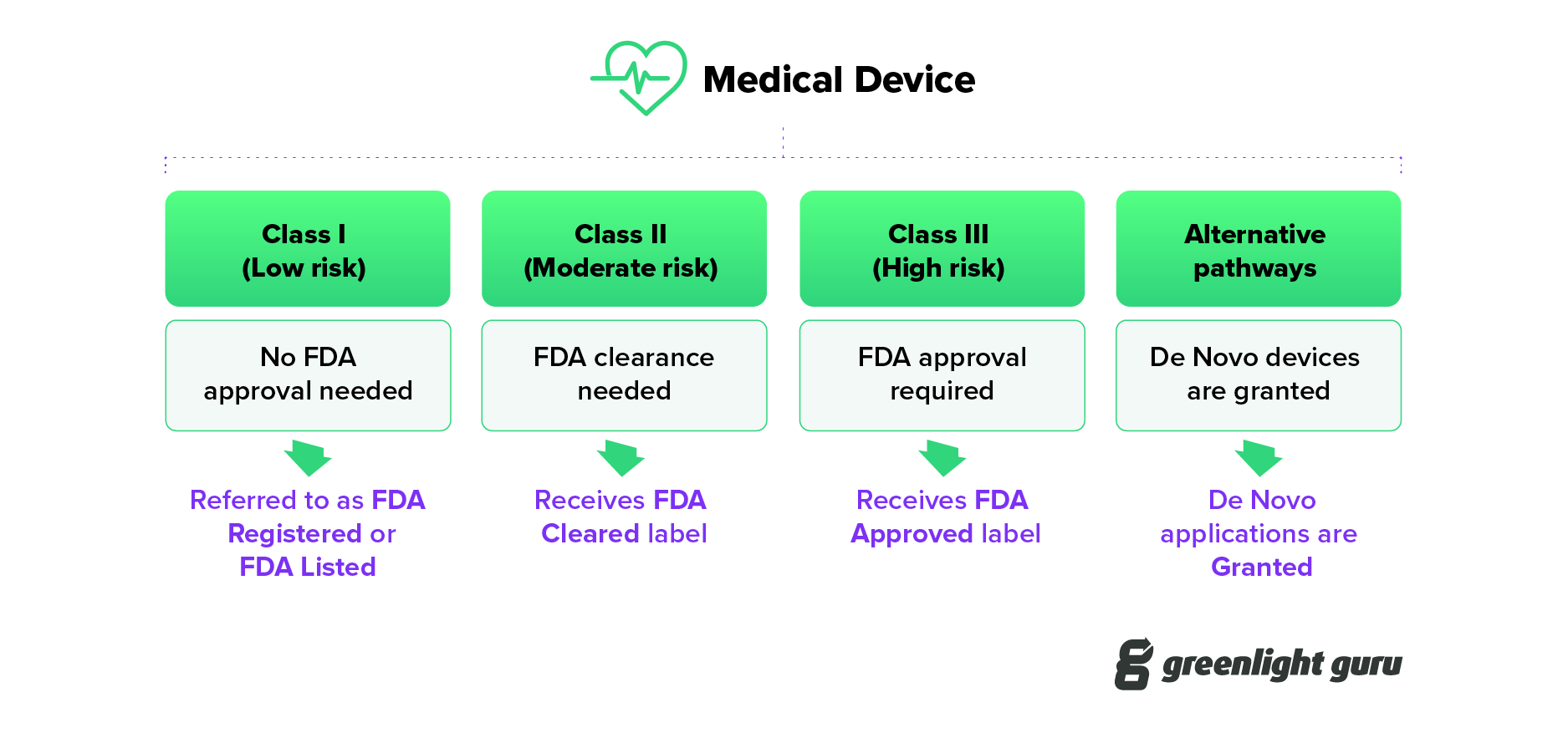
FDA Cleared vs. Approved vs. Registered: The Difference

The Difference Between FDA Registered, Cleared, Granted, Authorized and Approved
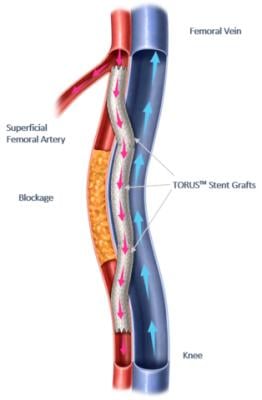
Endologix Receives FDA Approval of the DETOUR System to Treat Long Complex Superficial Femoropopliteal Lesions in Patients with PAD
Breakthrough Devices Program

FDA Device Regulation: 510(k), PMA · Academic Entrepreneurship for Medical and Health Sciences
What it Means to be Focused on Quality vs. Compliance

FDA Clearance Granted for First AI-Powered Medical Device to Detect All Three Common Skin Cancers

FDA Listed vs. Cleared vs. Approved: What's the difference?

What's The Difference: FDA Cleared Vs FDA Approved

What it Means to be Focused on Quality vs. Compliance

What's The Difference: FDA Cleared Vs FDA Approved

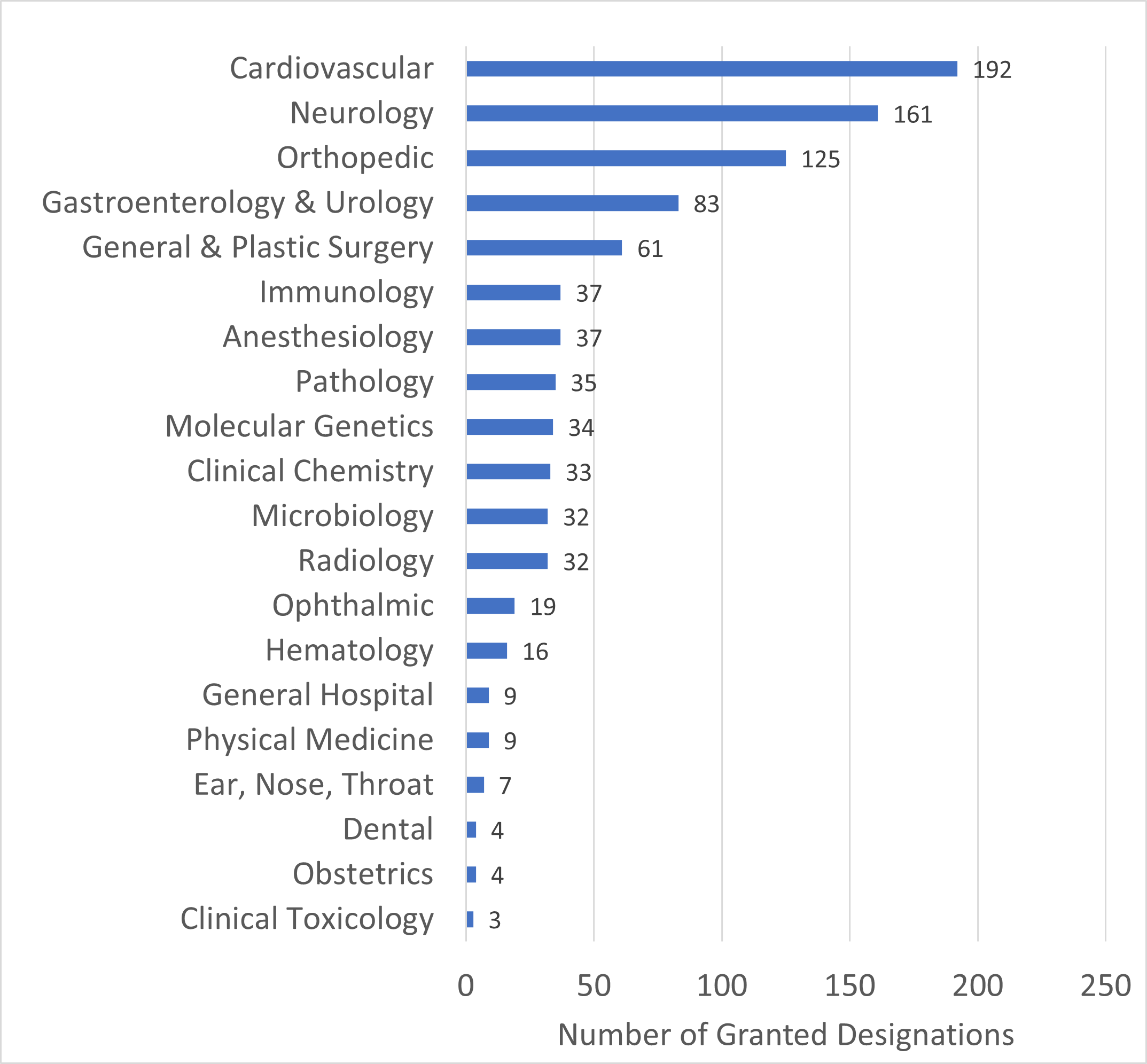
The Current State Of Almost 700 FDA-Approved, AI-Based Medical Devices

FDA Cleared vs FDA Approved • Advantu