Br2 - Diatomic Bromine Structure, Molecular Mass, Properties and Uses

By A Mystery Man Writer
Diatomic bromine is a chemical compound when two neutral bromine atoms combine by a covalent bond. Visit BYJU
Diatomic bromine is a chemical compound when two neutral bromine atoms combine by a covalent bond. Visit BYJU'S to understand the properties, structure and its uses.
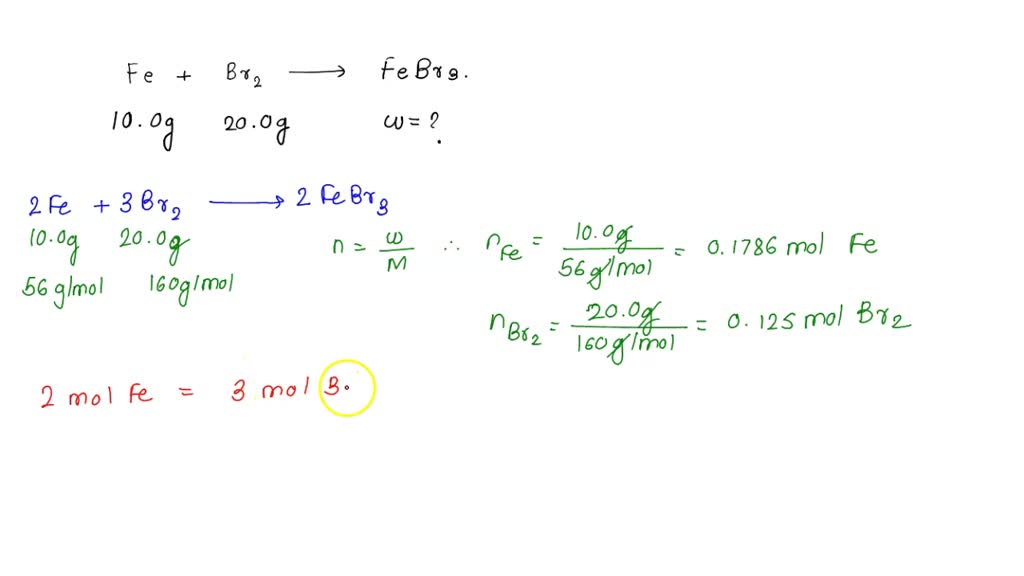
SOLVED: Iron can react with diatomic bromine to form iron(III) bromide. If a 10.0 g lump of iron were placed in 20.0 g of liquid bromine, what mass of iron(III) bromide would

Bromine has two naturally occurring isotopes (Br-79 and Br-81) an

Bromine, Structure, Properties & Uses

Bromine Formula: Learn Definition, Formula, Structure and Uses

Diatomic molecule - Wikipedia
Common Questions About the Bromine Water Test By Unacademy

Young Woman Portrait Back View Of Long Hair Stock Photo Download Image Now Red, Highlights Hair, Dyed Hair IStock

Hydrogen bromide
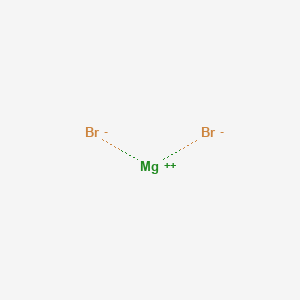
Magnesium bromide (MgBr2), Br2Mg
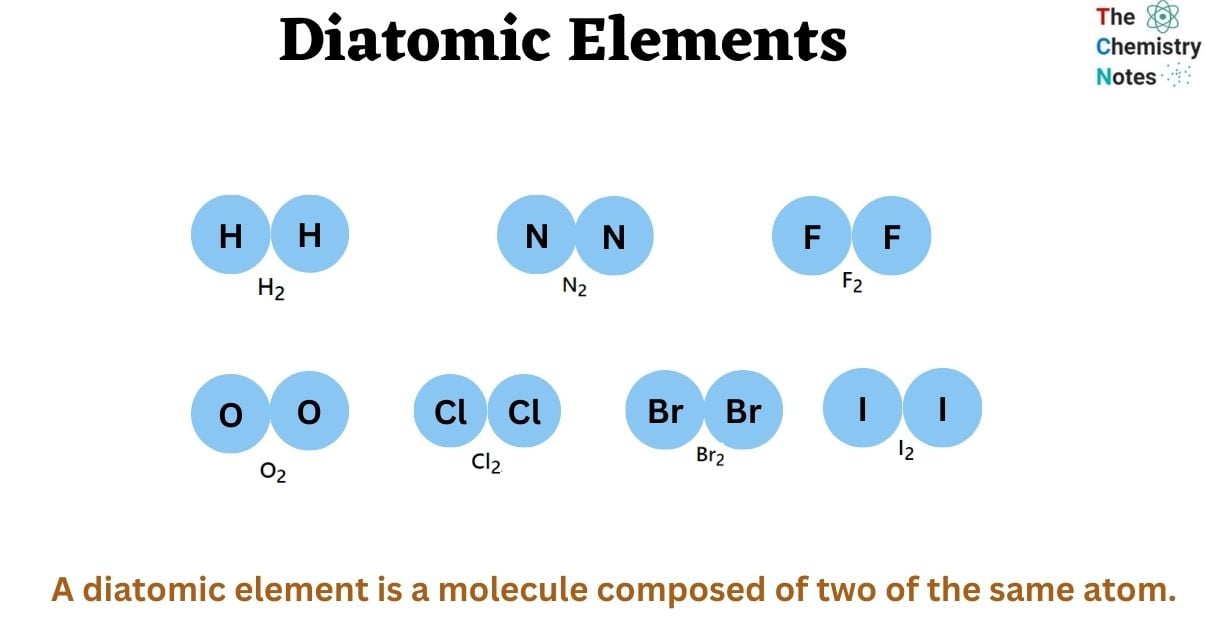
Diatomic Elements: Important 7 Elements properties formation

Group 7: Halogens GCSE Chemistry Revision

a Formation of a bromine molecule from dissociative adsorption of two

Bromine reagent grade 7726-95-6

SOLVED: a. What is the atomic mass of bromine? b. What is the molecular mass of bromine in its elemental form?