Microbial Culture Media For Quality Control Of Non-Sterile Products

By A Mystery Man Writer
lt;p>Using the correct media is critical to ensure microbiological quality. Explore a portfolio of culture media and substances for sample preparation, microbial enumeration tests, and tests for specified microorganisms.</p>
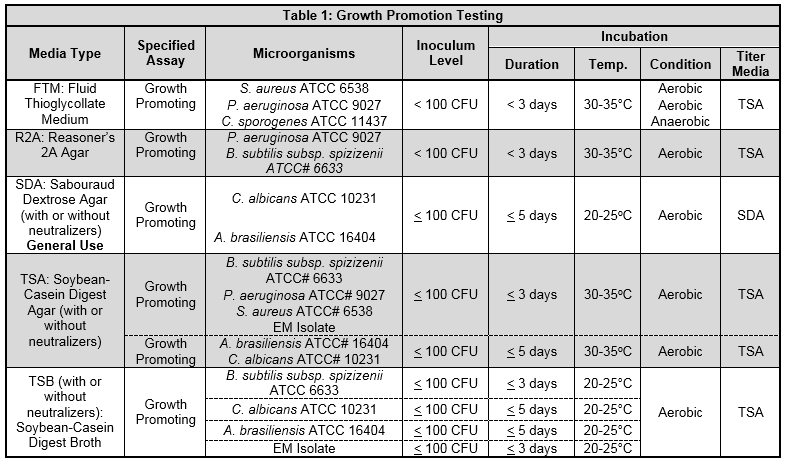
How To Establish Growth Promotion Tests For Pharmaceutical Culture Media

vertassets.blob.core.windows.net/image/e181af88/e1

PDF) Microbiological quality of non – sterile pharmaceutical products

Culture Media Pioneering Diagnostics

Without Measurement, There is No Control of Aseptic Processes

General Purpose Media : Types, Composition, Preparation, and Uses : A Comprehensive Guide

Prepared Culture Media Information

USP Testing for Non-Sterile Pharmaceutical Products: Achieving Gold in Analytical Excellence - Microbac Laboratories
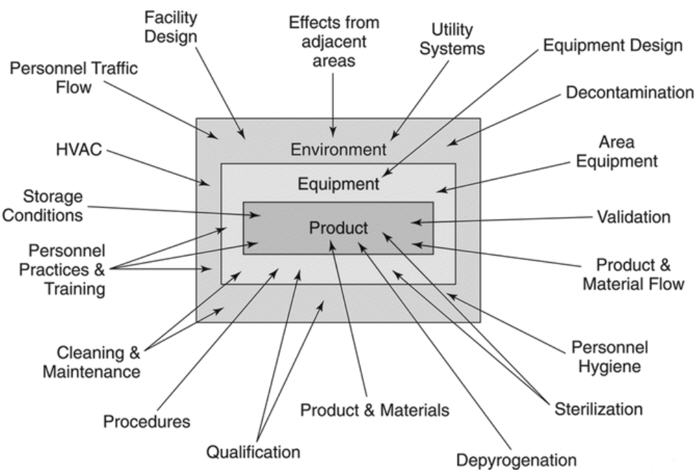
The Essential Components Of A Sterility Assurance Program

MilliporeSigma

Refining Microbiological Control for Non-Sterile Products
Microbiology Series: Water Activity
Fungal contamination of pharmaceutical products: a growing menace

How To Establish Growth Promotion Tests For Pharmaceutical Culture Media

Quality Control in Culture Media Preparation: Best Practices and Challenges
- Organic Cotton Comforter, Ivory Color

- Sexy Floral Lace Lingerie Set Sheer Unlined Bra Mesh Panties - Temu Canada

- Fajas - Ropa de compresión poscirugía para mujer, faja completa, body sexy con faja moldeadora (color piel, talla: M) : Ropa, Zapatos y Joyería

- Fine Yarn Bundle - 2/17s Merino Lambswool – The Oxford Weaving Studio

- Mount Airy Win Loss Statement Form - Fill Out and Sign Printable