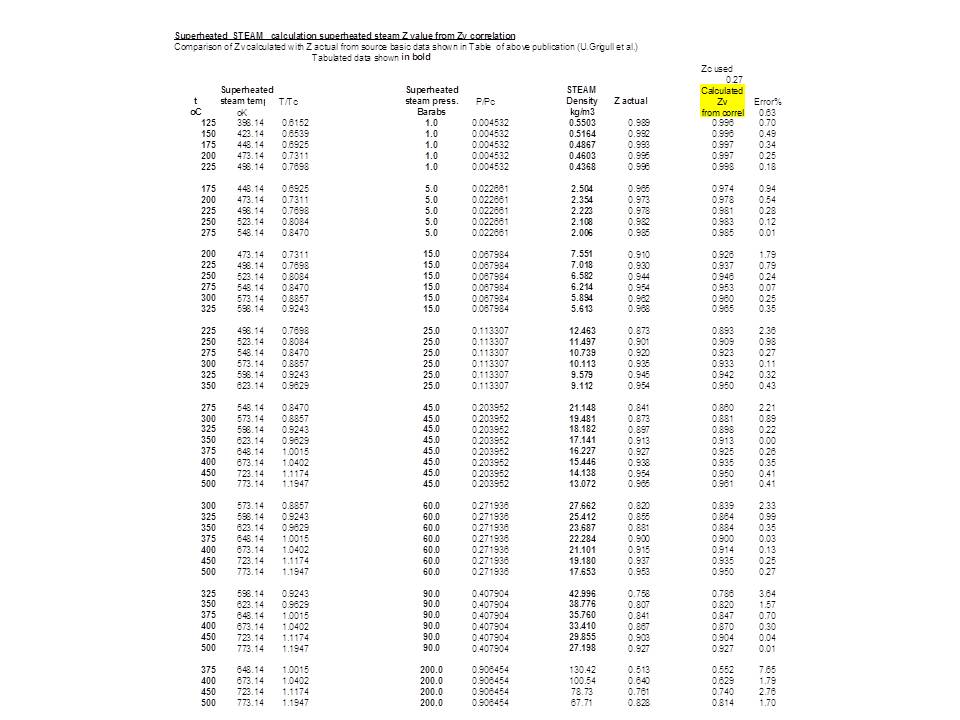
Graph of Compressibility Factor (Z) versus Pressure (Atm

By A Mystery Man Writer
Graph of Compressibility Factor (Z) versus Pressure (Atm)
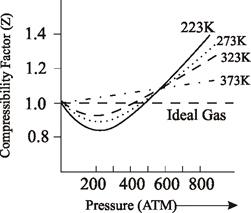
Chemistry Desk: Effect of Temperature

Compressibility factor - Wikipedia

Pressure, Physics Bilder – Durchsuchen 11,170 Archivfotos, Vektorgrafiken und Videos

Non-ideal behavior of gases (article)

The given graph represents the variation of Z(compressibility factor =displaystyle frac{mathrm{P}mathrm{V}}{mathrm{n}mathrm{R}mathrm{T}}) versus mathrm{P}, three real gases mathrm{A}, mathrm{B} and C. Identify the only incorrect statement.For the gas C
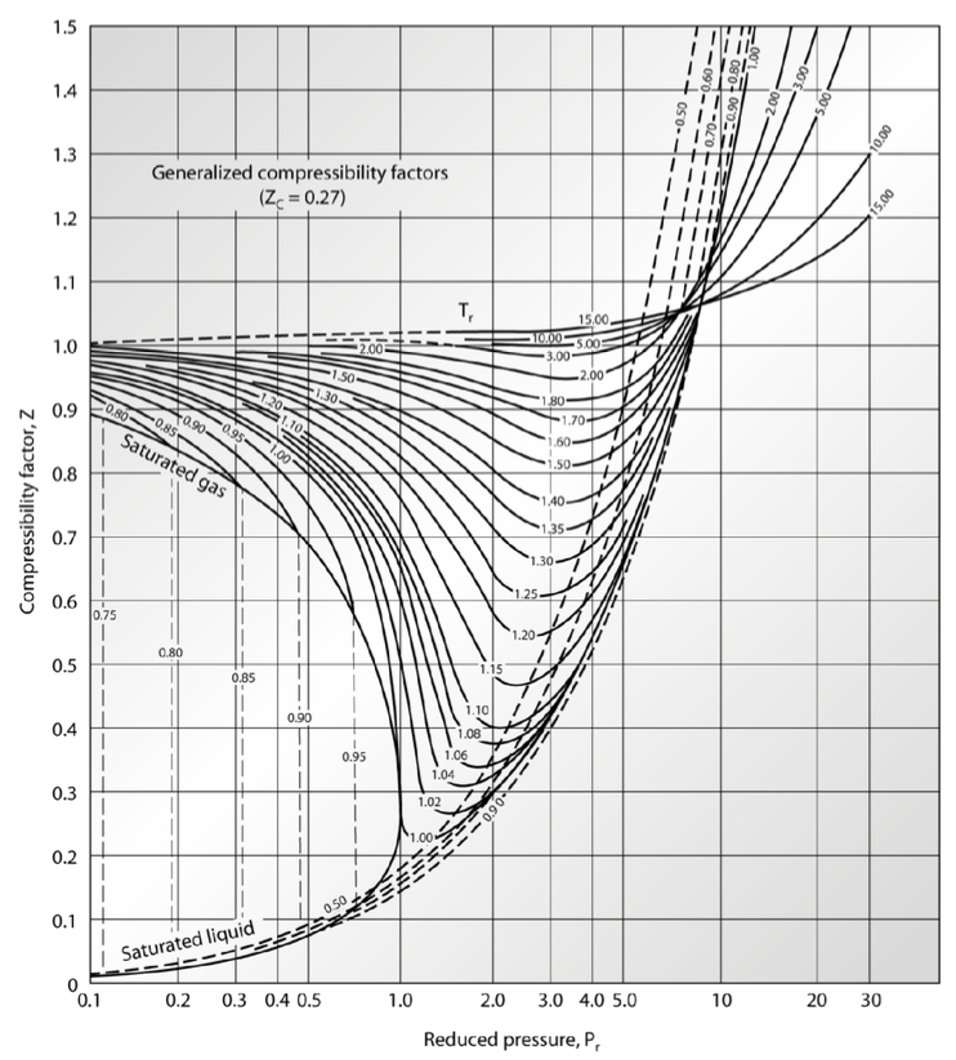
Qin Lab - thermal data

The graph of compressibility factor (Z) vs. P for one mole of a

Compressibility factor Z = PV / nRT is plotted against pressure as shown below:What is the correct order for the liquefiability of the gases shown in the above graph? A. CO 2
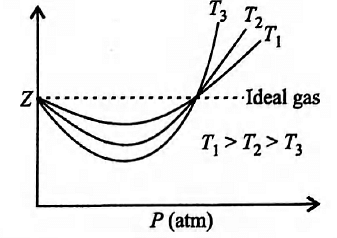
Boyle?? temperature or Boyle point is the temperat
- 5 Tips for Finding the Best Bra After Breast Reduction Surgery

- 1,900+ Coffee Sizes Stock Illustrations, Royalty-Free Vector

- Enamor Women's Stretch Cotton Antimicrobial Beginners Slip-on

- Why a Silicone Wedding Band is the Perfect Choice for Adventurous

- Calvin Klein Men's Cotton Classics Crew Neck T-Shirt