Ideal Gas Law: Doubling Temperature and Volume
By A Mystery Man Writer

A sample of neon gas has its volume doubled and its temperature held constant. What will be the new pressure relative to the initial pressure? What is the amount of the volume

SOLUTION: The gas laws unit test review and test questions and answers scored - Studypool

Principal of gases, gas law flow of

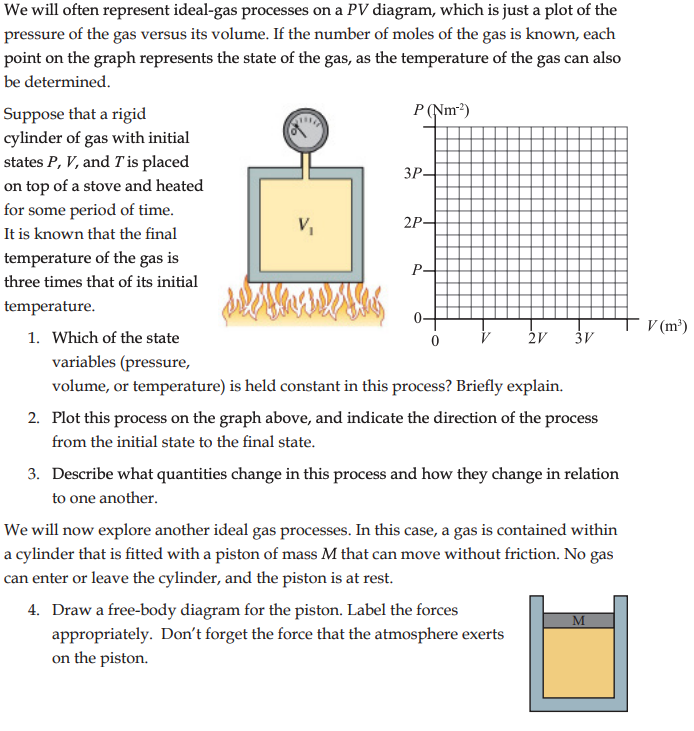
Worksheet for Exploration 20.3: Ideal Gas Law The

How to Calculate a Final Temperature Using the Ideal Gas Law Equivalency, Physics
:max_bytes(150000):strip_icc()/143058853-56a12f375f9b58b7d0bcdc3c.jpg)
Charles' Law Example Problem

The ideal gas law – why bubbles expand if you heat them, Physics

THERMODYNAMICS Day 7 of ppt video online download

SOLVED: Doubling the initial pressure, at constant temperature under which 1000 mL of a gas was confined causes the volume of the gas to decrease very slightly. According to Boyle's Law, the
tx95p2a.GIF
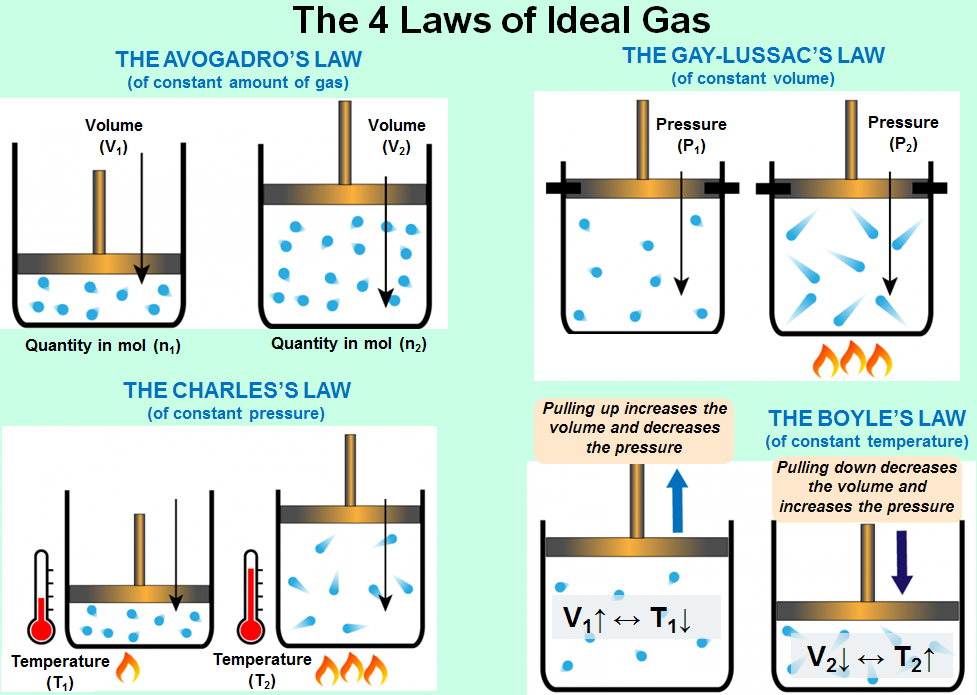
THE 3rd STATE OF MATTER – The 4 Laws of Ideal Gas – Computer Aided Design & The 118 Elements
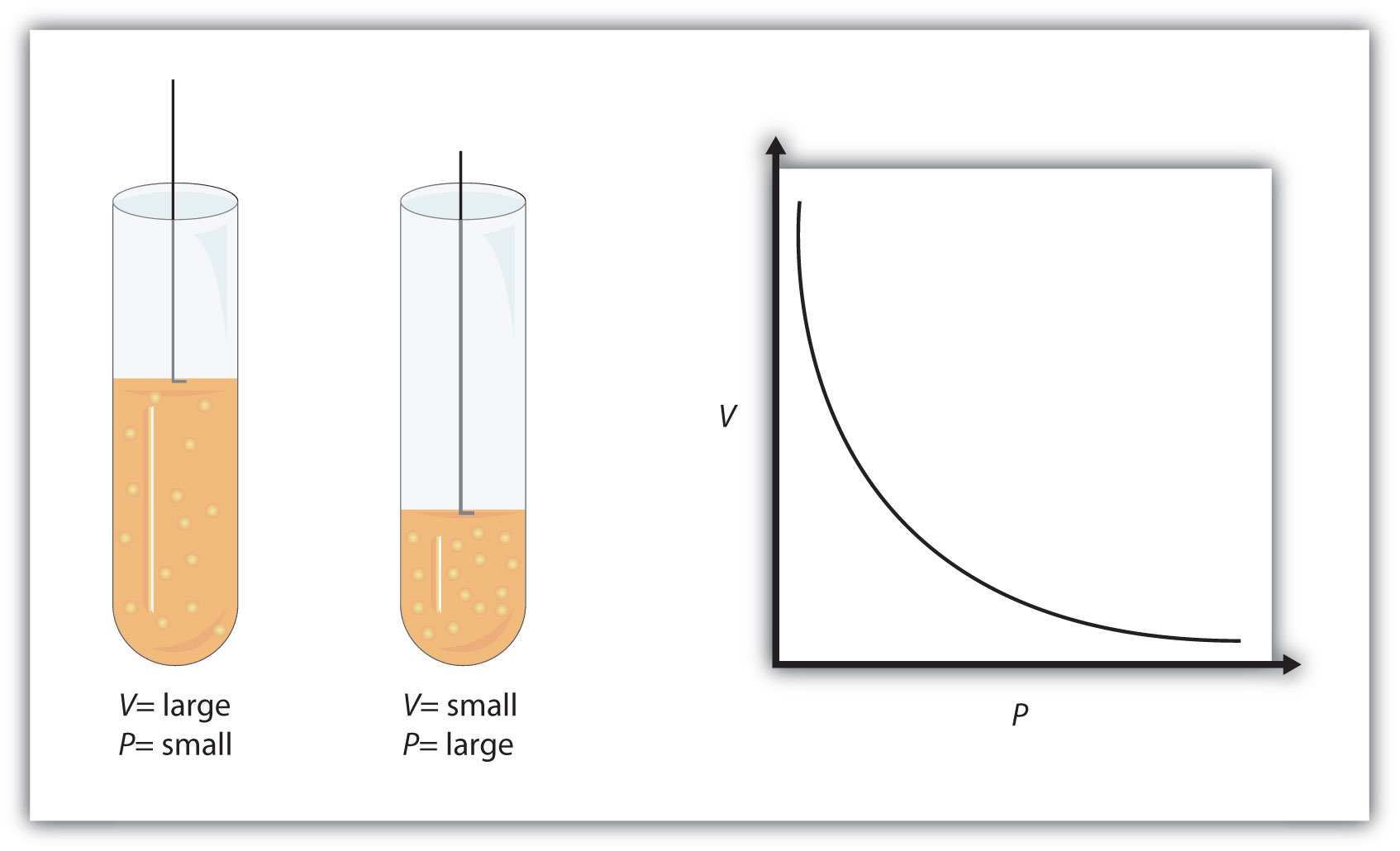
Gas Laws

Gas Laws: Boyle's Law, Charle's Law, Gay-Lussac's Law, Avogadro's Law