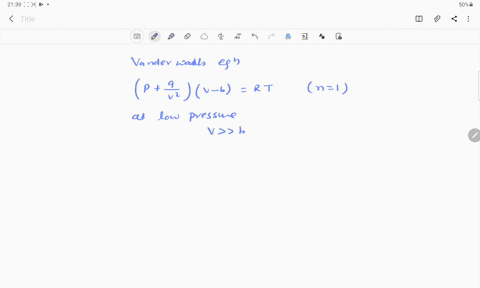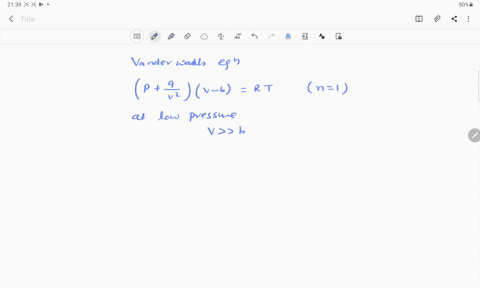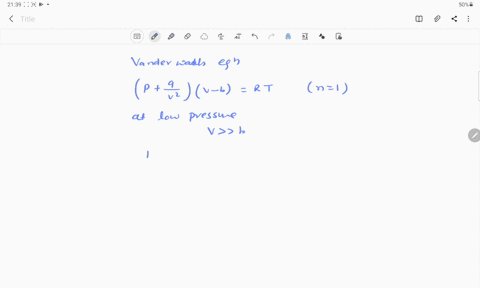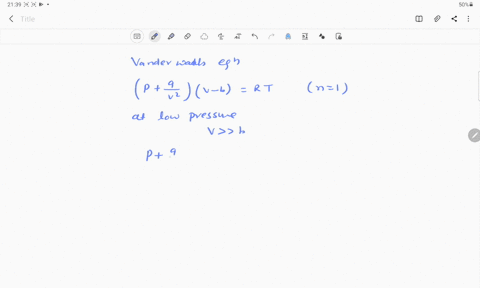
If Z is a compressibility factor, van der Waals equation at low pressure ..

By A Mystery Man Writer
Solution For If Z is a compressibility factor, van der Waals equation at low pressure can be written as
Video solution 1: If Z is a compressibility factor, van der Waals equation at low pressure can be written as
Video solution 2: If Z is a compressibility factor, van der Waals equation at low pressure can be written as
Video solution 3: If Z is a compressibility factor, van der Waals equation at low pressure can be written as
Video solution 4: If Z is a compressibility factor, van der Waals equation at low pressure can be written as

If Z is a compressibility factor, van der Waal's equation low

⏩SOLVED:If Z is a compressibility factor, van der Waals equation

physical chemistry - Why do some gases have lower value of Z for a

SOLVED: I need the answer as soon as possible. 16. If Z is a

For one mole of a Van der Waals gas when b=0 and T=300 K, the PV

Compressibility factor (Z) for a van der Waals real gas at

The given graph represent the variations of Z Compressibility

Solved APPENDIX Problem 1: Molar Volume and Compressibility

Bengali] At a low pressure, the van der waals equation reduces to (P+

⏩SOLVED:If Z is a compressibility factor, van der Waals equation
⏩SOLVED:If Z is a compressibility factor, van der Waals equation

Solved 2. (20 points) At low pressures, the compressibility

Compressibility factor (gases) - Citizendium
- Gas Compressibility Factor Calculator Excel SpreadsheetLow Cost Easy to Use Spreadsheets for Engineering Calculations Available at Engineering Excel Spreadsheets

- Non-Ideal Gas Behavior Chemistry: Atoms First

- At low pressure, the van der waal's equation is written as (P+ a/V^2)V=RT . Then compressibility factor is then equal to
- Compressibility Factor Calculator - Community
- What is the value of compressibility factor in terms of vander waal cons†an t at different conditions of pressure and volume?Why is Z>1 for H2 and He gas
- The Gorilla Grip Bed Sheet Strap Is the Best Hack for Securing Fitted Sheets

- Atacado Camiseta Masculina De Manga Comprida Compression Base Layer Tight Tops Under Skin T Shirt De $125,84

- all in motion, Pants, All In Motion Mens Large 3638 Black Midweight Thermal Pants W 4 Way Stretch

- Crystal Rhinestone Hoodie Strings - Rhinestone Rope - Rhinestone String – Be Createful

- 29 Tall, Heavy Duty, Single Folding Table Leg
