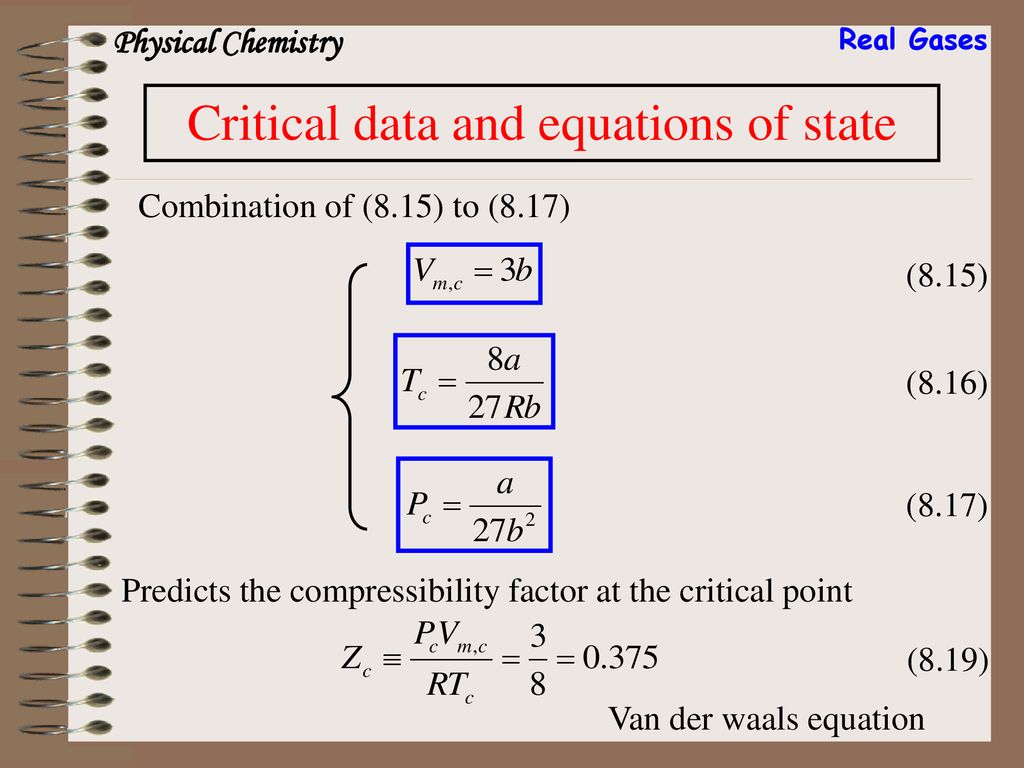
Real gases

By A Mystery Man Writer

Ideal Gases vs Real Gases – MCAT General Chemistry

How might the world be different if all gases behaved ideally? (Use photo to answer)

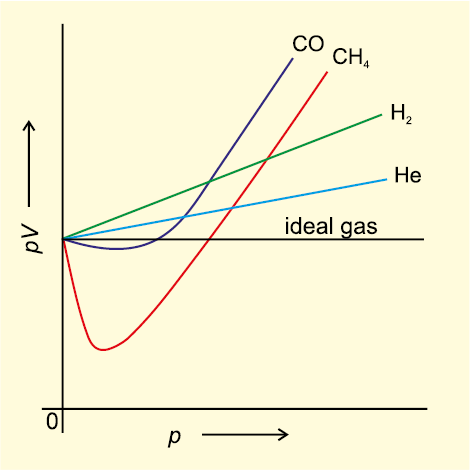
The given graph represents the variation of Z(compressibility factor =displaystyle frac{mathrm{P}mathrm{V}}{mathrm{n}mathrm{R}mathrm{T}}) versus mathrm{P}, three real gases mathrm{A}, mathrm{B} and C. Identify the only incorrect statement.For the gas C

The behavior of real gases in terms of reduced pressure.

REAL GASES, S. DE R.L. DE C.V.

The Deviation Of Real Gas From Ideal Gas Behavior - Practice Questions & MCQ

Ideal Gas Law Formula and Examples

17 Behaviour Gases Royalty-Free Images, Stock Photos & Pictures
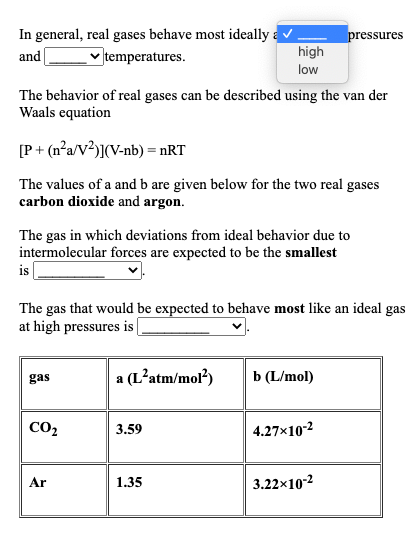
Solved In general, real gases behave most ideally a
Sections

Description of real gases: Compression factor

Chemistry 231 Real Gases. The ideal gas equation of state is not sufficient to describe the P,V, and T behaviour of most real gases. Most real gases depart. - ppt download

Compressibility factor (z): real gases deviate from ideal behav-Turito

SOLUTION: Real gases ex e vjcyadx - Studypool
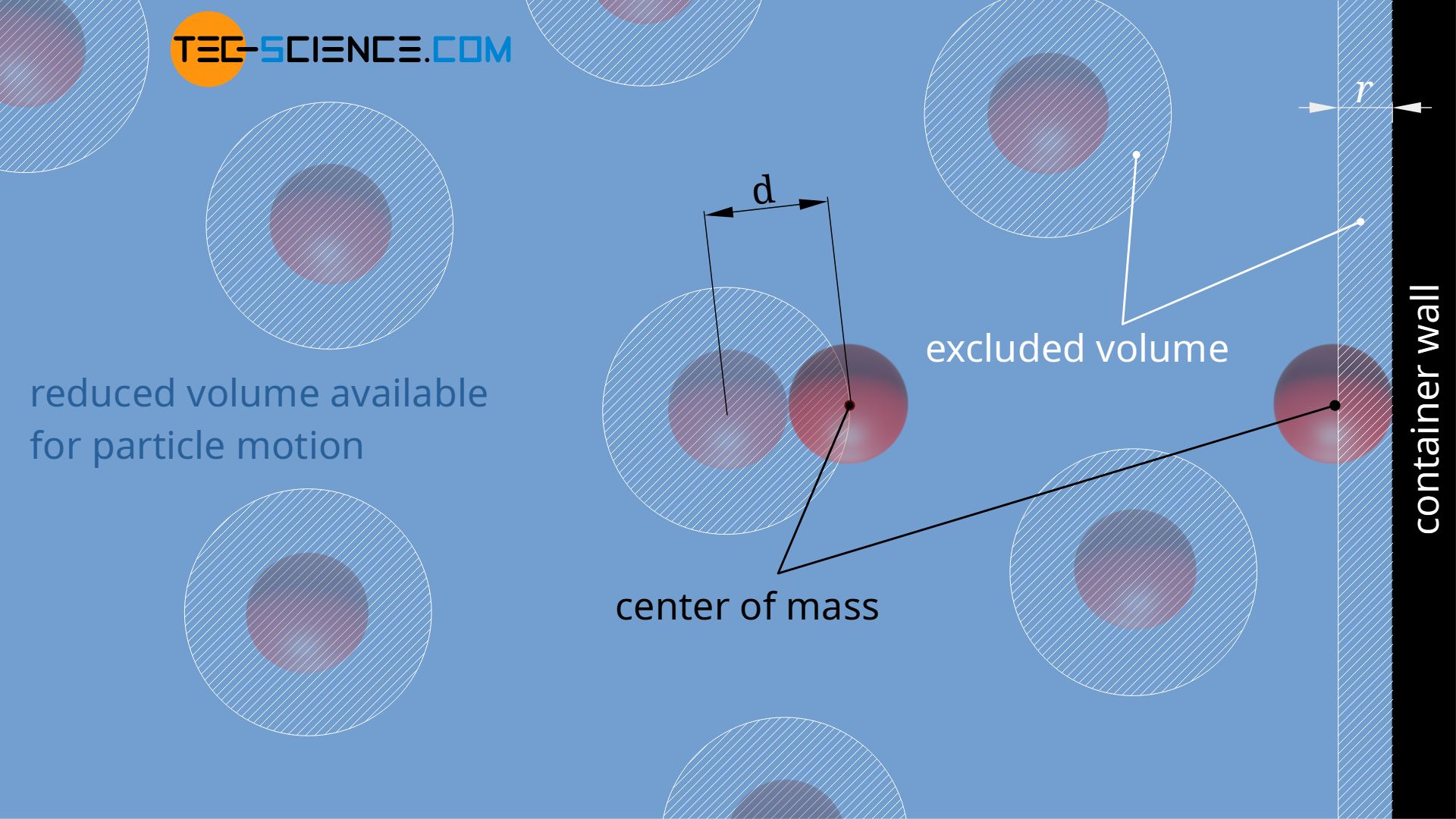
Van der Waals equation (gas law for real gases) - tec-science
- Lululemon athletica Wunder Train Contour Fit High-Rise Crop 23, Women's Capris

- MP Women's Impact Scrunch Seamless Leggings - Teal Blue

- Big Dot of Happiness Carnival - Step Right Up Circus - Lion

- Gator Stripping Pads - Multi-Surface - Green - 6-in L x 3 7/8-in W - 2 Per Pad 7324012

- Musculação para mulheres: especialistas respondem 3 principais