Solved RT B 2. The compressiblity factor for a gas is

By A Mystery Man Writer
Answer to Solved RT B 2. The compressiblity factor for a gas is
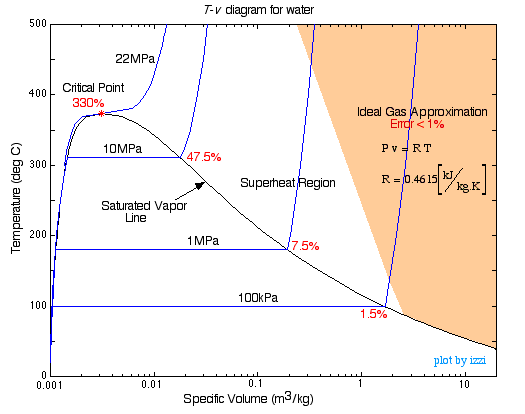
Chapter 2b: Pure Substances: Ideal Gas (updated 1/17/11)
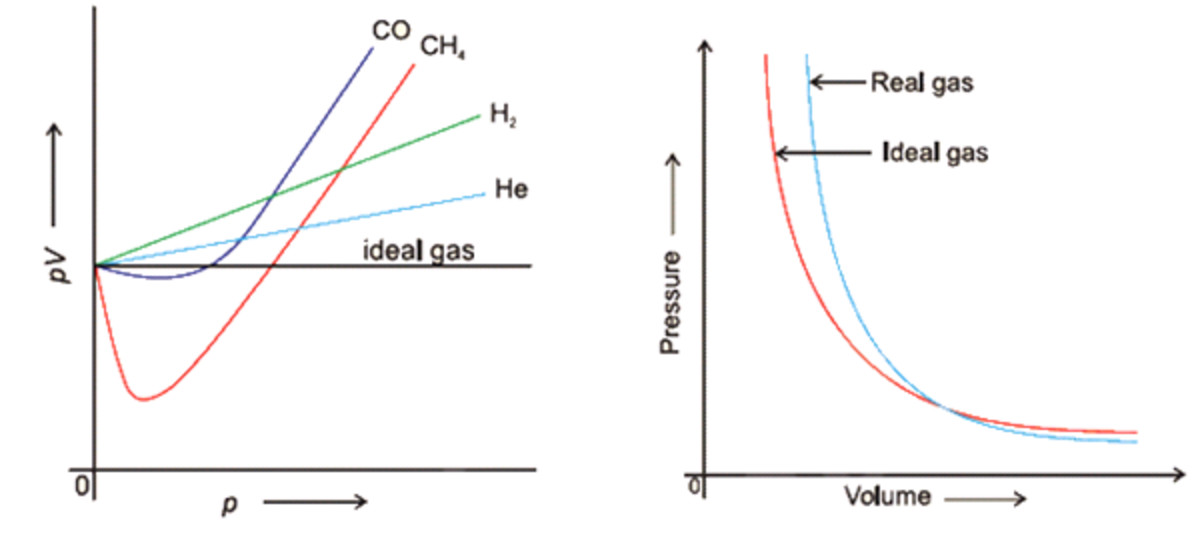
Real Gases - Chemistry, Class 11, States of Matter

The compressibility factor a real gas high pressure is:-1 - frac{Pb} {RT}1 + frac {RT} {Pb}11 + frac {Pb} {RT}

Find the isothermal compressibility `x` of a Van der Walls gas as a function of volume
What is the value of compressibility factor in terms of vander waal cons†an t at different conditions of pressure and volume?Why is Z>1 for H2 and He gas

Lecture 4-Real-Gases, PDF, Gases

Compressibility factor for methane.

OneClass: 2. Fugacity for a van-der-Waals gas Let's get a feel for how much fugacity deviates from pr
How can we calculate critical temperature, volume and pressure in terms of a and b? - Quora

Solved RT B 2. The compressiblity factor for a gas is

Deviation of Real Gases from Ideal Gas Behaviour - GeeksforGeeks
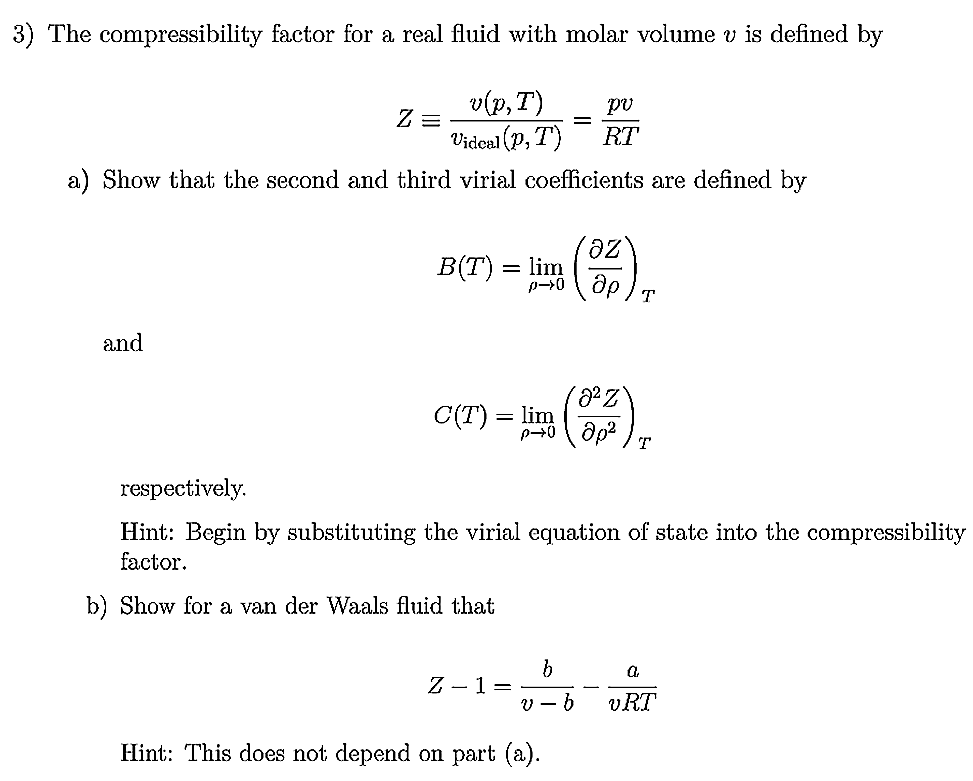
- Solved 3) The compressibility factor for a real fluid with
- Real gasses For an ideal gas, the compressibility factor Z = PV/nRT is equal to unity for all conditions. For a real gas, Z can be expressed as a function. - ppt

- Gas Compressibility Factor Calculator Excel SpreadsheetLow Cost
- What is the compressibility factor (Z) for 0.02 mole of a van der

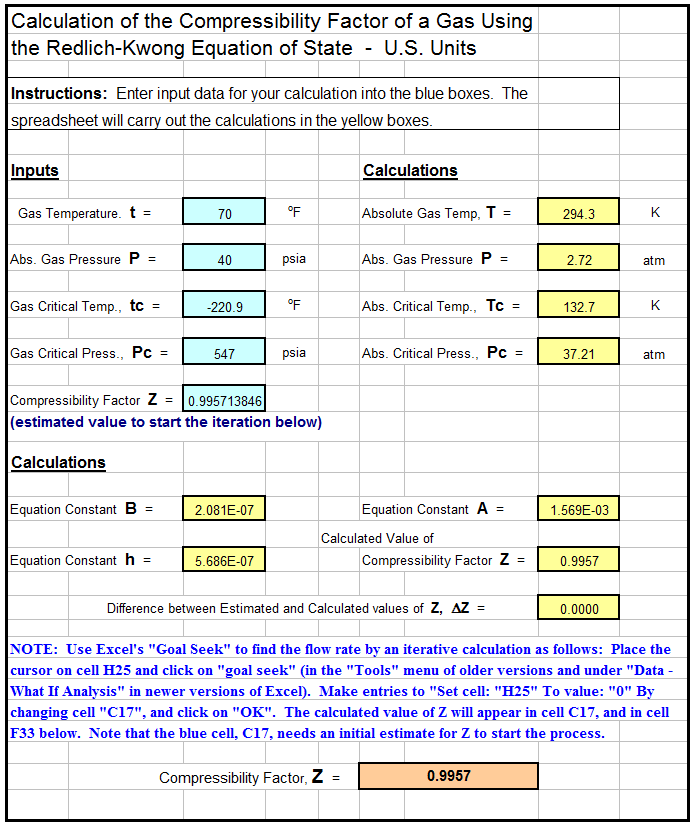
- Math cad compressibility factor, z, of real gas using the redlich

- Brazil Fotbollströja Neymar JR

- Now Trending in Fashion Editorials: This Sheer Fendi Pre-Fall 2020 Gown - Fashionista

- Steampunk Steel-Boned Lace-Up Overbust Corset

- Shop high caliber TYR Men's Triathlon Tops. Carbon Tri Top and

- Leggings Tights Women Push Up Sports Legging Pocket High Waist Exercise Trousers Running Fitness Gym Leggings Femme Yoga Pants - AliExpress
