Solved What is the equilibrium constant (Kp) at 45 °C for

By A Mystery Man Writer
Answer to Solved What is the equilibrium constant (Kp) at 45 °C for

Pressure Equilibrium Constants (Kp)

The equilibrium constant (KP) of the reactions N2O4hArr 2NO2 was found

The equilibrium constant (K_p) of the reaction N_2O_4 rightleftharpoons 2NO_2 was found to be 636mm 49.7^oC. Calculate the percentage dissociation of N_2O_4 when the pressure of the gas mixture is 182mm.
Chemistry Kp MCQ - The Student Room
The equilibrium constant (K) for the reaction,2SO2(g)+O2(g)2S03(g) at 1000 K is 3.5 atmWhat would be the partial pressure of oxygen gas,if the equilibrium is found to have equal moles ofSO2 and SO3?

Solved 11) Suppose a system operating in accordance with the

The equilibrium constant for the interconversion of PCl_5 and PCl_3 is 0.0121: PCl_5 PCl_3 + Cl_2. A vessel is charged with 0.123 mol PCl_5. At equilibrium what is the concentration of PCl_3?

Calculating Equilibrium Constants (Part I)

Chapter 14
A complex equilibrium question
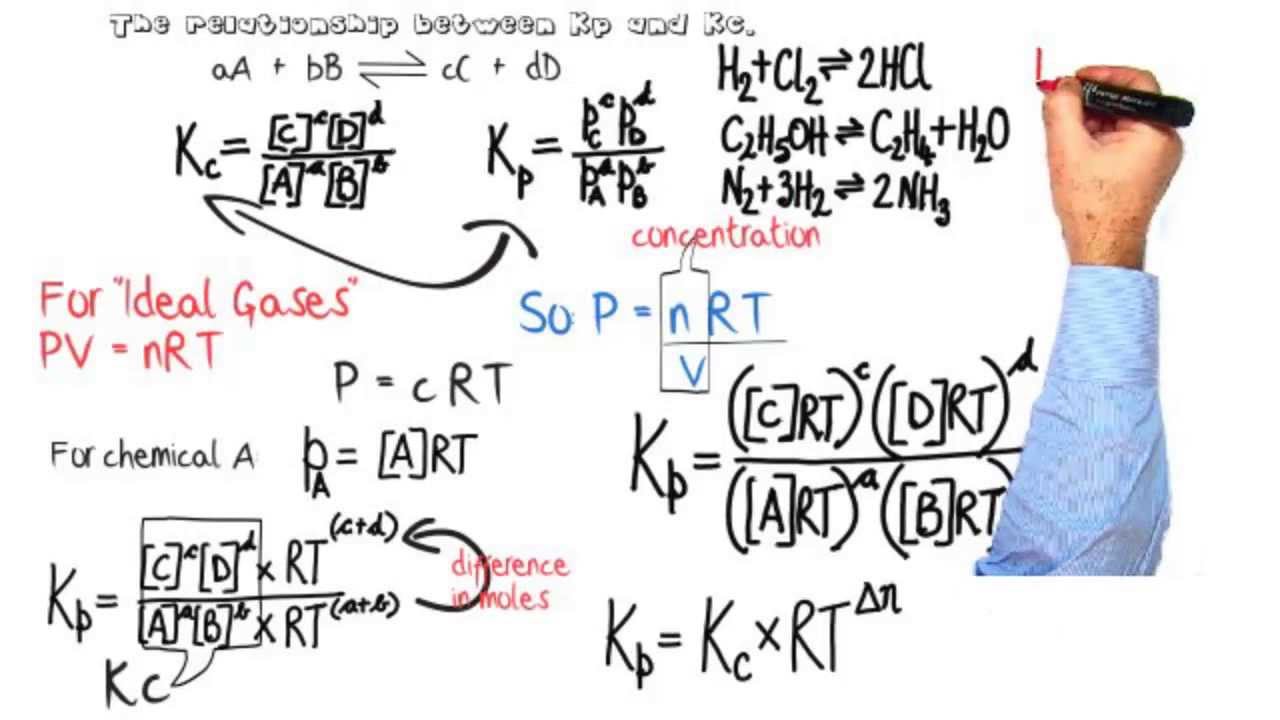
Equilibria: Relationship between equilibrium constants Kp & Kc.
- A solution is to be kept between 40°C and 45°C. What is the range of temperature in degree fahrenheit - Sarthaks eConnect
- 12 Pcs UL KSD301 125V 16A Thermal Switch,Circuit Off at 45°C/113°F and On at 35°C/95°F Normally Closed NC Microwave Thermostat,Auto Reset Bimetal

- VeloEdge TUBE 700X35/45C F/V 48MM REMOVABLE

- North American JRB-45C, Detail of JRB-45C nose. (U.S. Air F…

- Toyo-View 45C Large Format Field Film Camera with Case, Rodenstock Bundle 411082010076

- Lace Triangle Bralette Everyday Bralettes Comfy Bralette Thin Padded Bralette Y2k Bralette

- Fitness Woman High Impact Sport Bra Plus Size XXL Cross Straps Wirefree Adjustable Buckle Nylon Yoga Underwear Gym Workout Bra 01 Pink 2XL

- Bulkey Code Wallet - Merlot, Safe, - Bulkey Code Wallet

- XOXO Hot Pink & Black Satin Bra & Matching India

- Ombre Seamless Bra Lorna Jane - Active Living
