Write the expression for the compressibility factor (Z) for one mole of a gas. Write the value of Z for an

By A Mystery Man Writer

6.3: Van der Waals and Other Gases - Physics LibreTexts

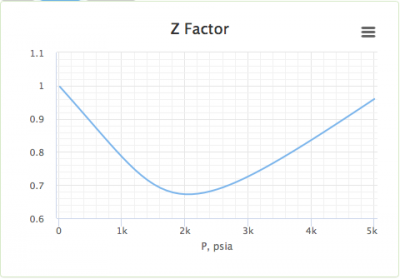
Gas Compressibility - an overview

Compressibility Factor - an overview

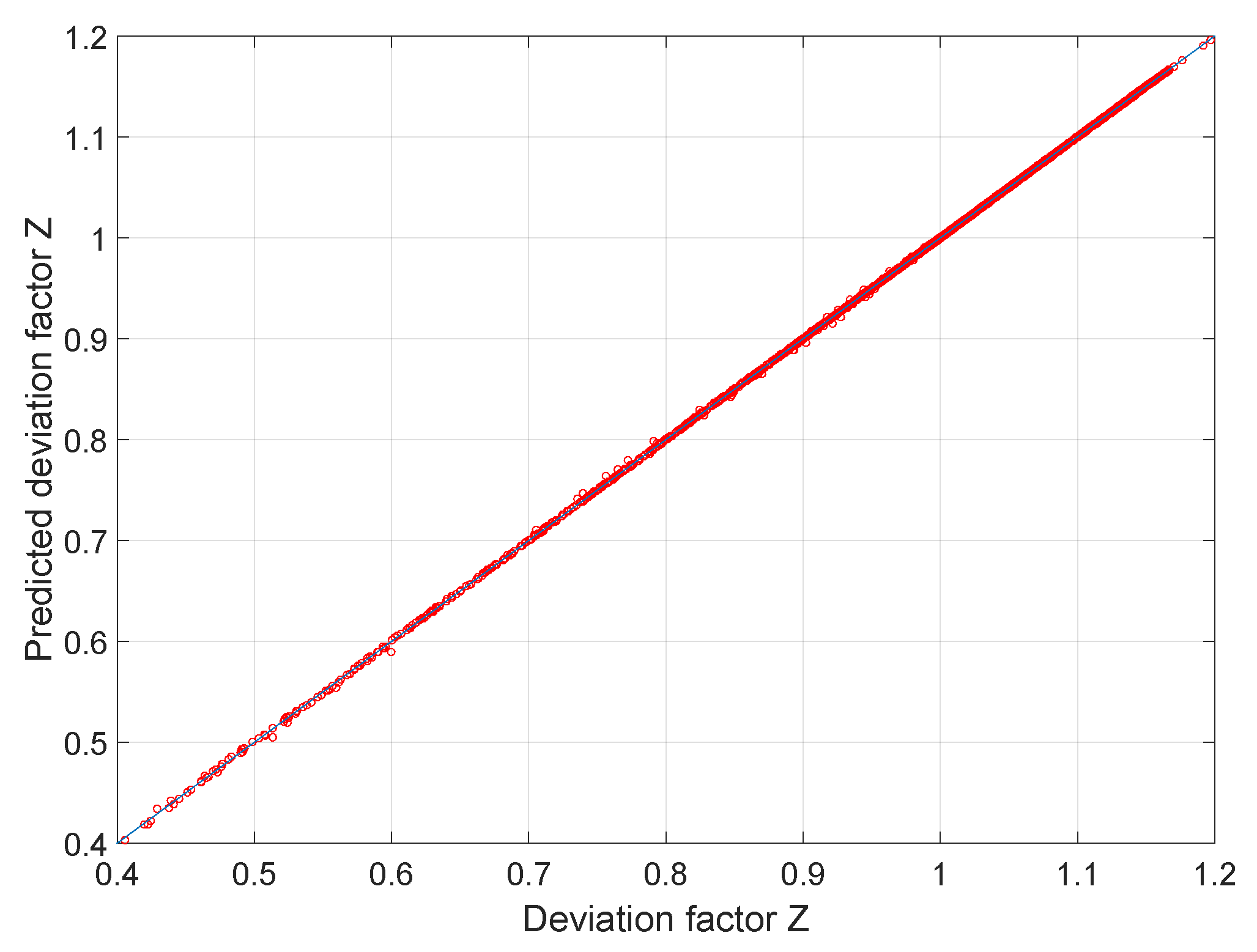
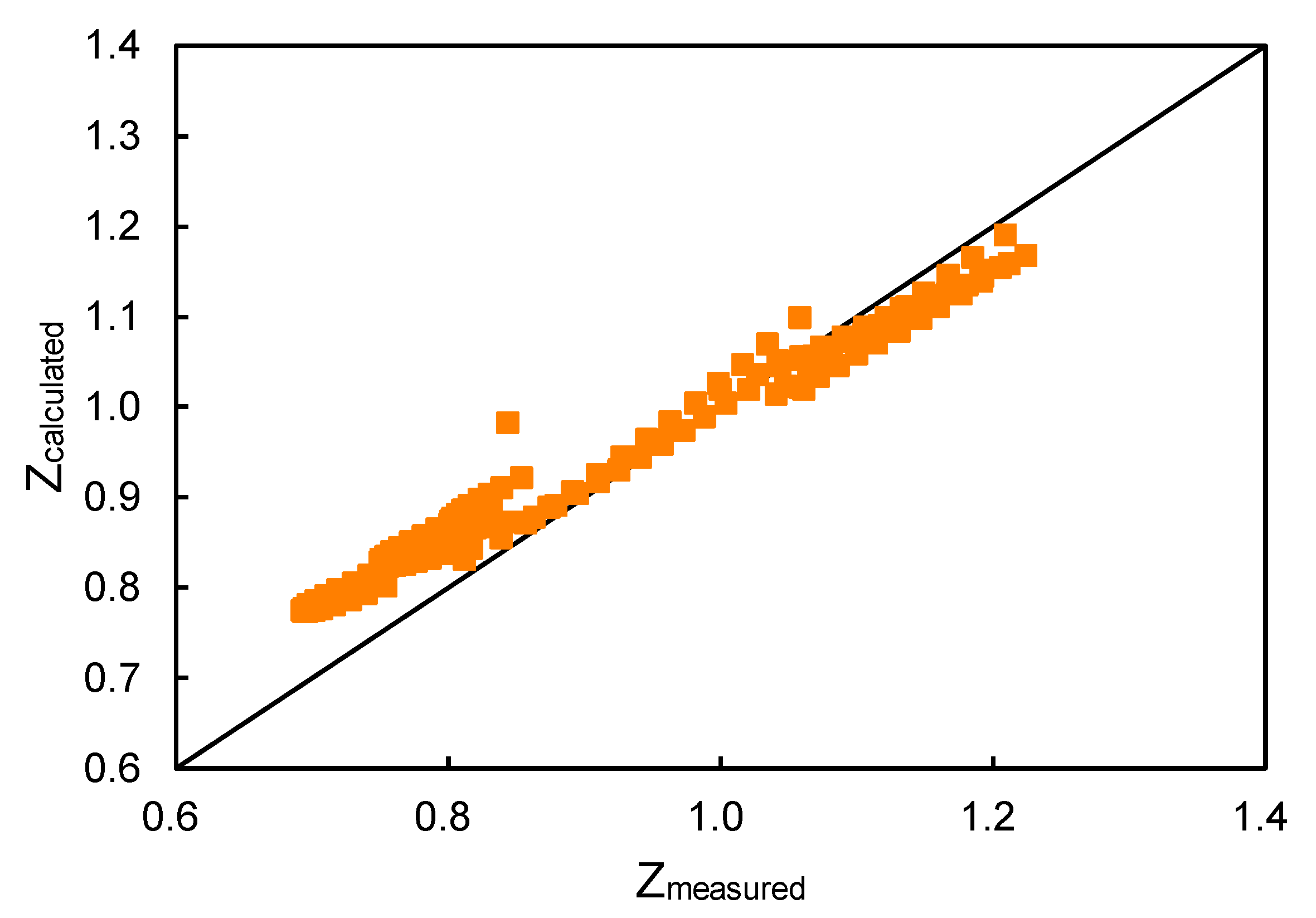
Compressibility Factor Calculator

Welcome to Chem Zipper.com: The compressibility factor for 1 mole of a van der Waals gas at 0oC and 100 atm pressure is found to be 0.5. Assuming that the volume of

The compressiblity factor Z for 1 mole of a real gas at low pressure can be written as

Compressibility factor - Wikipedia

Sheet - 01 - Real Gas, PDF, Gases
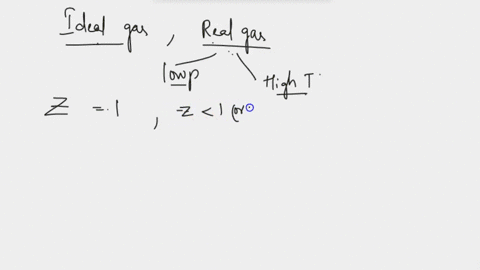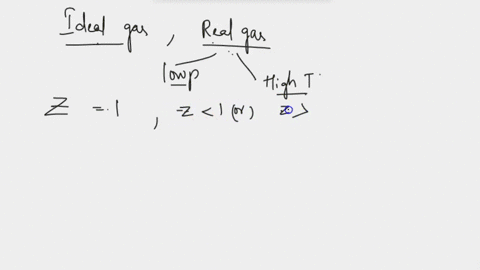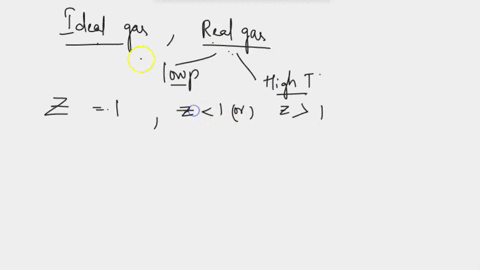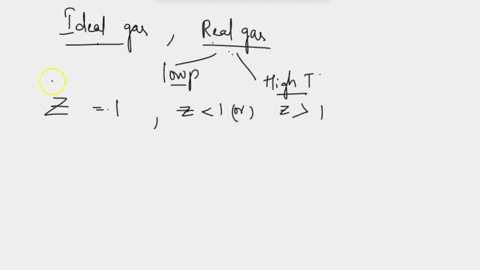
SOLVED: Derive the mathematical expression expressing the compressibility factor Z of a real gas depending on the reduced variables; Explain in detail how the volume of the actual gas at a given

Deviation from ideal gas behaviour
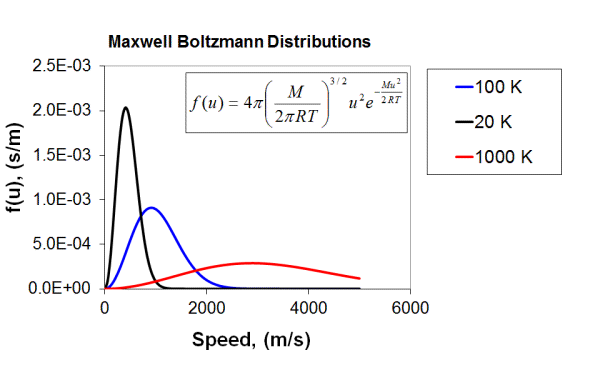
Gas Laws – First Year General Chemistry

Gujrati] Explain compressibility factor (Z).

SOLVED: Derive the mathematical expression expressing the compressibility factor Z of a real gas depending on the reduced variables; Explain in detail how the volume of the actual gas at a given
- Separatec esporte desempenho masculino bolsa dupla boxer perna

- Virginia Metalcrafters Brass Beehive Candlestick 7 Tall – Harvin

- Floral Print Briefs Comfy Breathable Stretchy Intimates - Temu

- Vera Natura Women Baggy Cargo Pants Low Rise Wide Leg Sweatpants Casual Multi Pockets Loose Parachute Pants Jogger Trousers

- Captain Underpants Confirmed for Marvel's Phase 4 – Marvelous Movies