The Cottrell Experiment and Diffusion Limitation 3/3

By A Mystery Man Writer
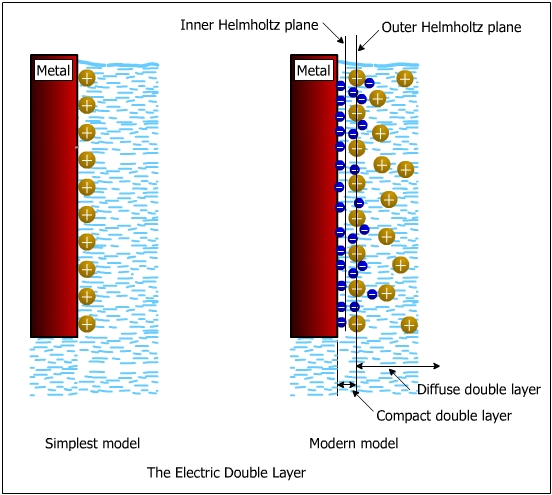
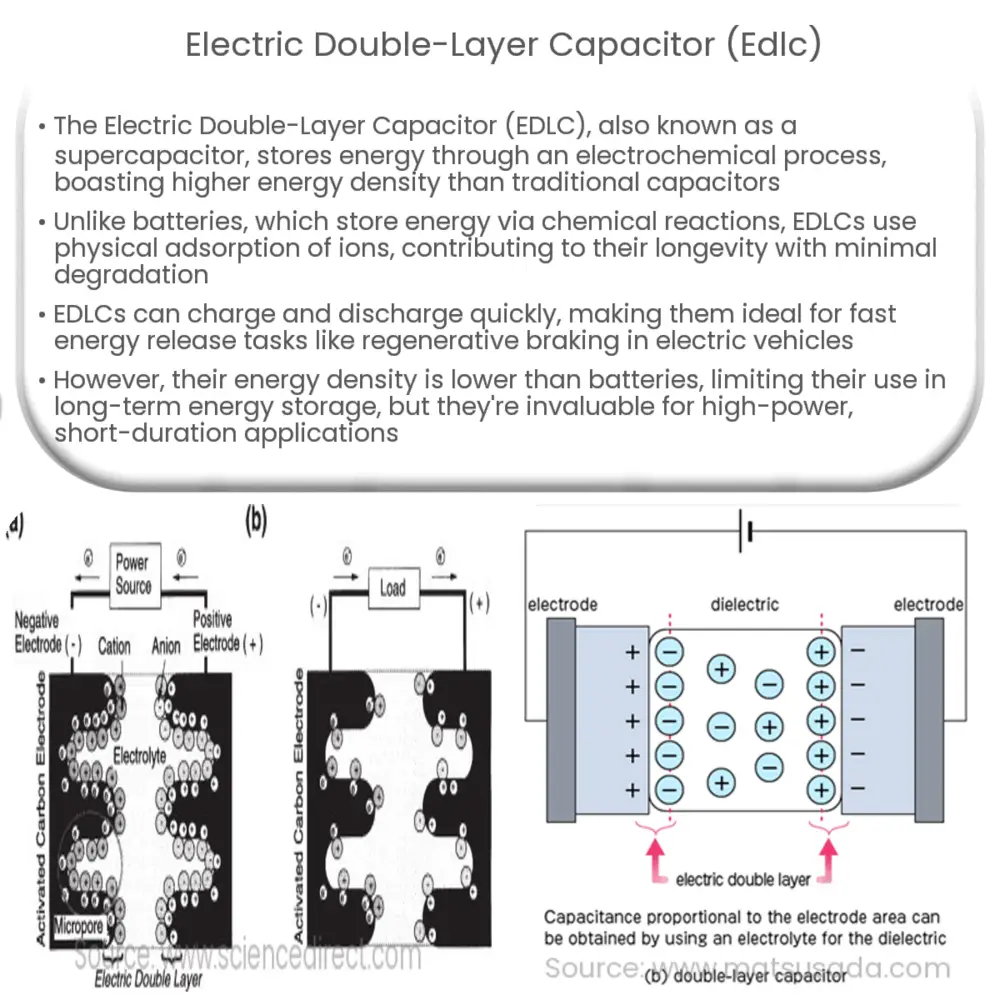
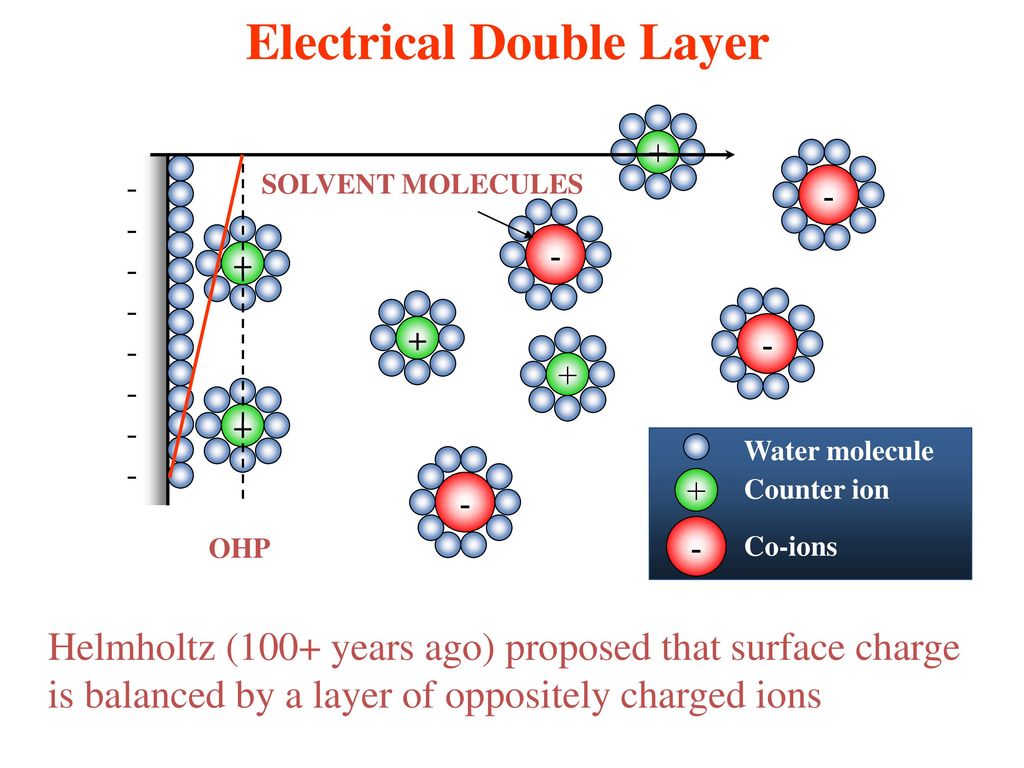
In this chapter the electrochemical double layer and its features are discussed. The electrochemical double layer acts as a capacitor and every change in the potential of the electrode will induce a capacitive charging current that is caused by physics not by a chemical reaction. This current decays exponentially.

PDF) Comparison between Cottrell diffusion and moving boundary models for determination of the chemical diffusion coefficients in ion-insertion electrodes
Crystals, Free Full-Text

Polymers, Free Full-Text

Chapter 3 transport phenomena in electrolytic systems and concentration overpotential. - ppt video online download
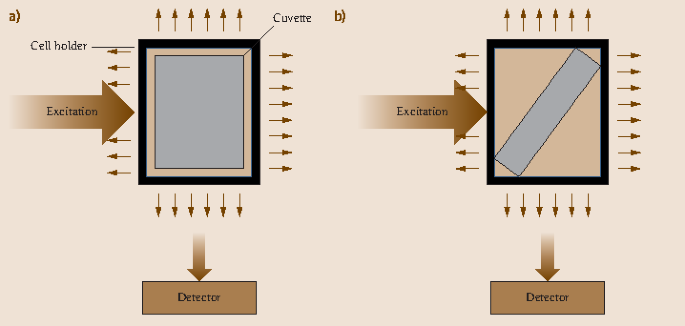
Spectroscopy of Electrochemical Systems
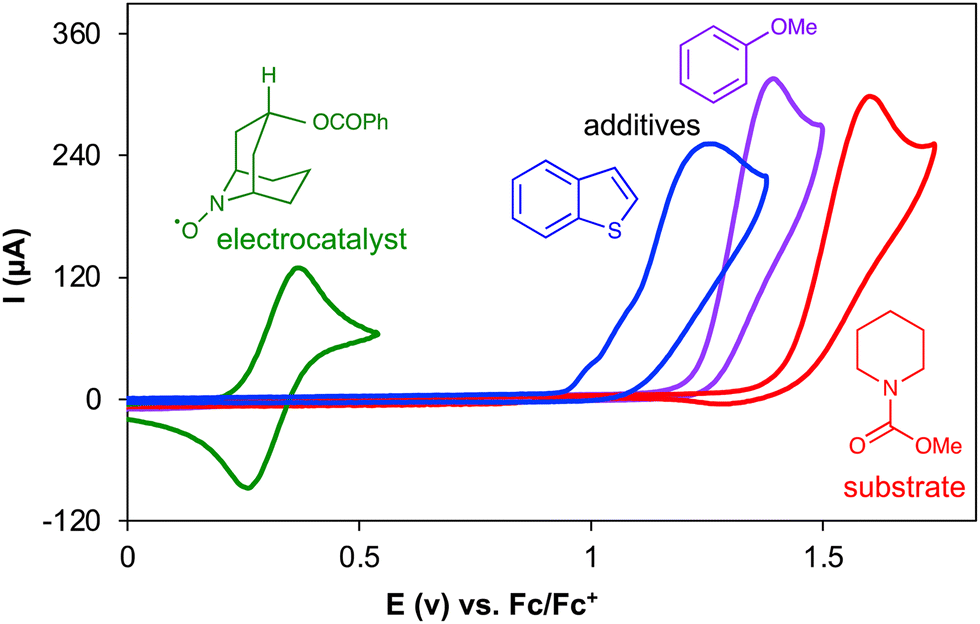
Cyclic voltammetry and chronoamperometry: mechanistic tools for organic electrosynthesis - Chemical Society Reviews (RSC Publishing) DOI:10.1039/D2CS00706A

How to optimize the analytical performance of differential pulse voltammetry: one variable at time versus Design of Experiments
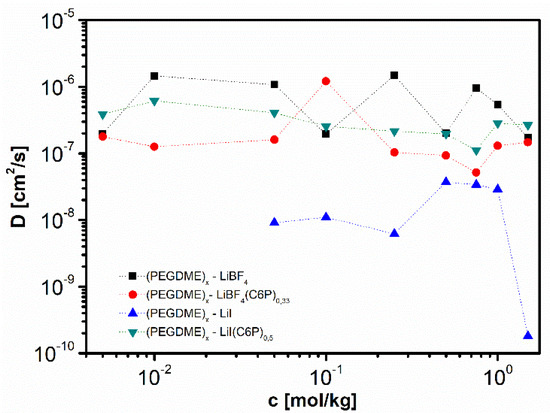
Polymers, Free Full-Text

Fluorinated ether decomposition in localized high concentration electrolytes - ScienceDirect

Electrochemical-Shock Synthesis of Nanoparticles from Sub-femtoliter Nanodroplets

Phase Transformation Lecture 3
Theory - Chemistry LibreTexts

Cottrell equation - Wikipedia
- New Zumba Jogger Pants Women Clothes For Teenagers Korean Style

- Shapermint: Shaper shorts

- Three Different Uses of Tracksuits Tracksuit, Competition outfit, Clothes

- 3PC Knee Brace Set Built-in Full Leg Compression Sleeve Knee Pain Relief & Patella Stabilizer Knee Brace Support for Hiking Soccer Basketball Running Jumpers Knee Tennis Volleyball & Squats Black-A Large

- Hollister Men Jacket Casual Leisure Windproof Grey size S