At a given temperature T gases Ne Ar Xe and Kr are found to deviate from ideal gas behavior (JEE MAINS 2019) - Doctor Logics Sunny Garg Chemistry

By A Mystery Man Writer
At a given temperature T, gases Ne, Ar, Xe and Kr are found to deviate from ideal gas behavior. Their equation of state is given as P=RTV−b at T. Here, b is the van der Waals constant. Which gas will exhibit steepest increase in the plot of Z (compression factor) vs P?

Solved In general, real gases behave most ideally at

Advances in Energy Research, Vol. 1: Suneet Singh Venkatasailanathan Ramadesigan Editors, PDF, Photovoltaics

Solved 4.9 The ideal gas law, pu = RT, describes the

At a given temperature T gases Ne Ar Xe and Kr are found to deviate from ideal gas behavior (JEE MAINS 2019) - Doctor Logics Sunny Garg Chemistry
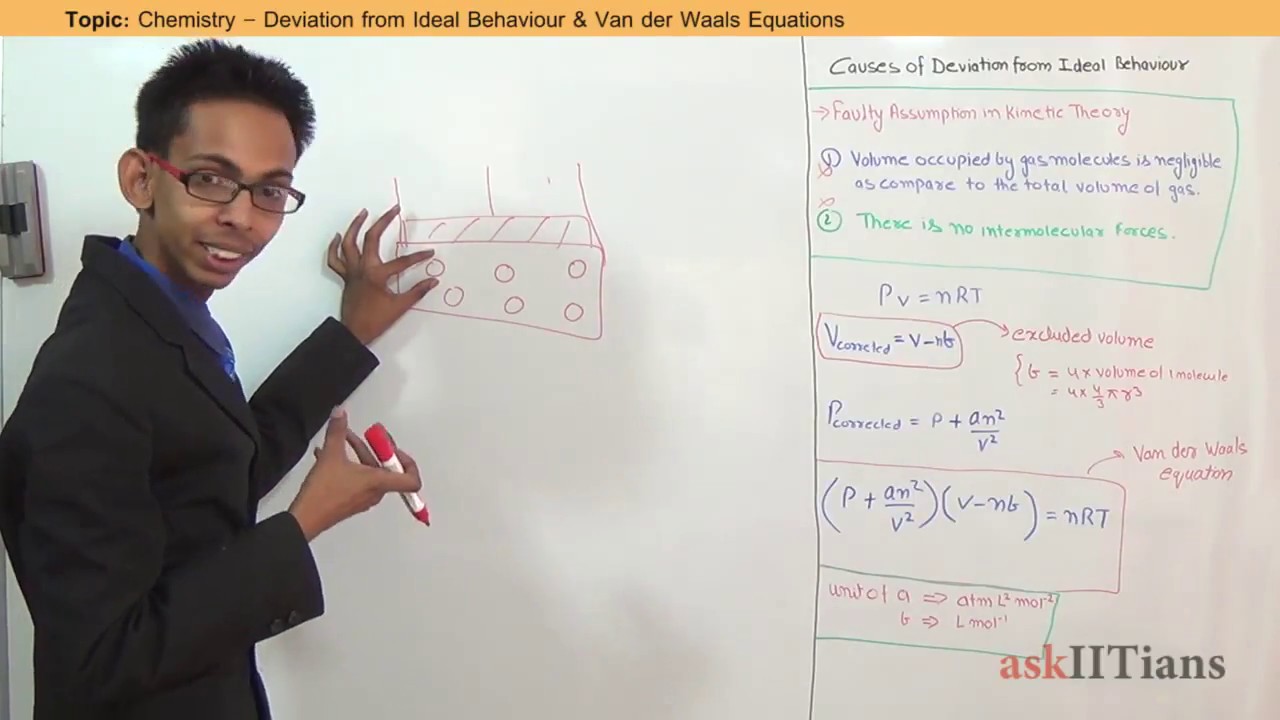
Deviation from Ideal Behavior & Van der Waals Eqn, Chemistry, 11th, IITJEE Main/Adv., NEET

At a given temperature T gases Ne Ar Xe and Kr are found to deviate from ideal gas behavior. jee

Modern Techniques in Biosensors Detection Methods and Commercial Aspects (Gorachand Dutta, Arindam Biswas Etc.), PDF, Biosensor

At a given temperature T, gases Ne, Ar, Xe and Kr are found to deviate
At a given temperature T gases Ne Ar Xe and Kr are found to deviate from ideal gas behavior (JEE MAINS 2019) - Doctor Logics Sunny Garg Chemistry

The temperature of an ideal gas is increased from 27∘ C to 127∘ C. Then, percentage increase in V rms isA. 37 %B. 11 %C. 33 %D. 15.5 %

A given sample of an ideal gas occupies a volume V at a pressureand absolute temperature T. The m
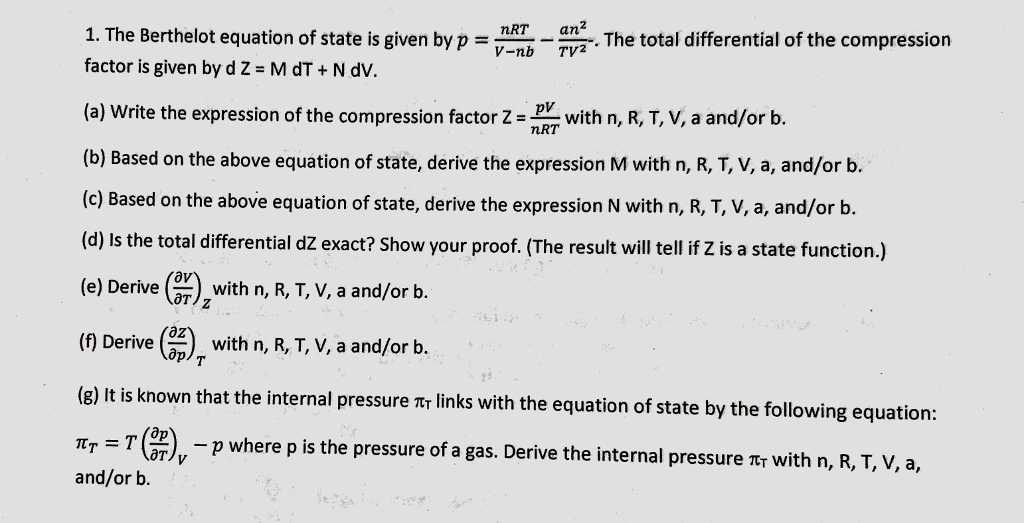
- Solved The Berthelot equation of state is given by
- Solved 1. The compression factor, Z of a gas is 0.625. Which
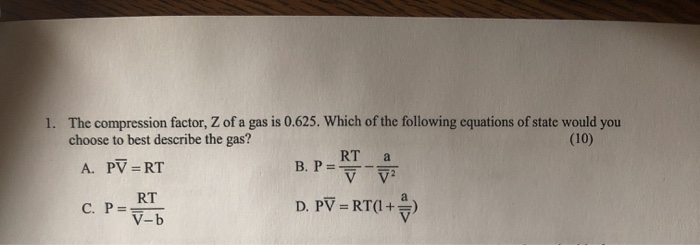
- At 273 K measurements on argon gave B = -21.7 cm$^3$ mol$^{

- Real Gases. The ideal gas equation of state is not sufficient to describe the P,V, and T behaviour of most real gases. Most real gases depart from ideal. - ppt download

- Solved a) b) c) State (i) the ideal gas equation (ii) the