What is the compressibility factor (Z) for 0.02 mole of a van der Waal

By A Mystery Man Writer
(d) (0.1+(1000xx(0.02)^(2))/(V^(2)))V=20xx0.02 =0.1V^(2)-0.4V+0.4=0 =V^(2)-4V+4=0 implies" "V=2L Z=(PV)/(nRT)=(0.1xx2)/(20xx0.02)=0.5

atm. 6. What is the compressibility factor (Z) 0.02 mole of a van der Waals' gas pressure of lam. Assume the size of gas molecules is negligible. Given : RT = 20

Compressibility factor - Wikipedia

Solved Show that the compressibility factor of van der Waals

The van der Waals parameters for gases W, X, Y and Z are {:(Gas,a(

or casts (B) decreases (C) remains same (D) changes unprediciduly B-16.What is the compressibility factor (Z) 0.02 mole of a van der Waals' gas pressure of 0.1 atm. Assume the size of

One way of writing the equation of state for real gases is PV = RT [1+

Filo Student Questions For CBSE , Grade 9

Welcome to Chem Zipper.com: The compressibility factor for 1
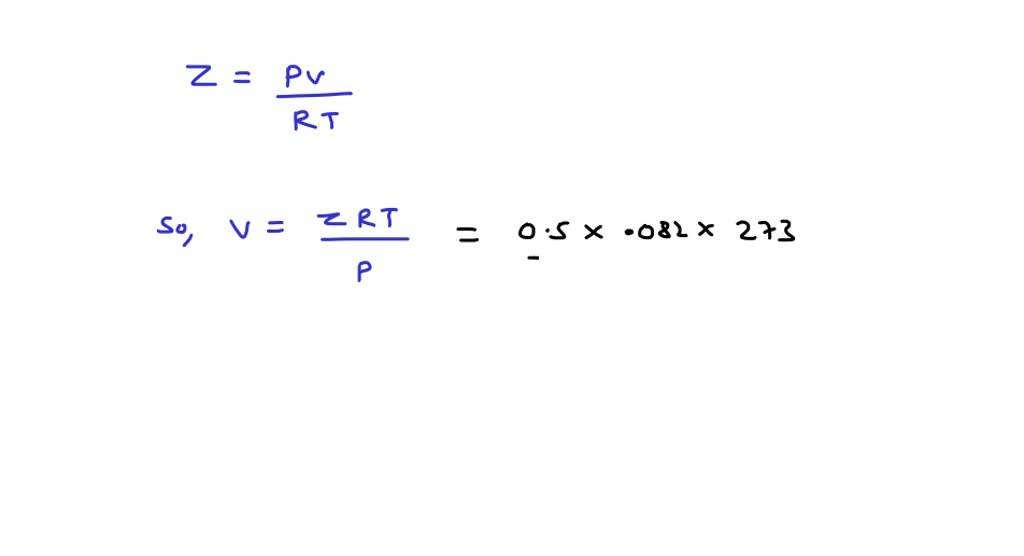
⏩SOLVED:Compressibility factor for 1 mol of a van der Waals gas
- At Critical Temperature,pressure and volume . The compressibility
- 3.2 Real gas and compressibility factor – Introduction to

- In the following compressibility factor Z vs pressure graph at 300 K, the compressibility of CH 4 at pressure

- Write the expression for the compressibility factor (Z) for one mole of a gas. Write the value of Z for an

- What is the value of compressibility factor in terms of vander
- Natori Bra 36C Underwire Mesh Overlay Molded Cups Adjustable Straps Lace Wings

- Blue mermaid bra. Outline mermaid top - t-shirt design. Scallop
- Underworks Men Magicotton Chest Binder Gynecomastia Compression

- Sanrio 15131120210130 Underwear My Melody 2-Piece Panties Girls Pink 130

- Warners Blissful Benefits Dig-Free Seamless Brief 3-Pack RS6333W
