At high pressure, the compressibility factor 'Z' is equal toa)unityb) c) d)ZeroCorrect answer is option 'C'. Can you explain this answer? - EduRev NEET Question

By A Mystery Man Writer

THREE STATES OF MATTER General Properties of Gases. - ppt download

The compressibility factor(s) for an ideal gas is/are: (A) unity

Solved 2. (20 points) Use the generalized compressibility
If assertion is true but reason is false.

1. The compressibility factor, z, is the ratio of
NEET Part Test - 1 Free MCQ Practice Test with Solutions - NEET

The compressiblity factor Z for 1 mole of a real gas at low pressure can be written as

The compressibility factor of an ideal gas is: 1. zero 2. infinite 3. 1 4. -1 NEET Practice Questions, MCQs, Past Year Questions (PYQs), NCERT Questions, Question Bank, Class 11 and Class 12 Questions, and PDF solved with answers

SOLVED: Hey guys, please help me friends. Choose the correct answer, don't say wrong answers. Select correct statement/s regarding compressibility factor Z of a gas: Z for an ideal gas is independent

1 The Ideal Gas. 2 Ideal gas equation of state Property tables provide very accurate information about the properties. It is desirable to have simple. - ppt download
Gaseous State Questions for JEE exam - Free Online All questions
- Physical Chemistry The Compression Factor (Z) [w/1 example]

- Compressibility factor, Z of a gas is given as Z= frac { pV }{ nRT } (i) What is the value of Z an ideal gas?(ii) For real gas what will be

- If Z is a compressibility factor, van der Waals equation at low pressure ..

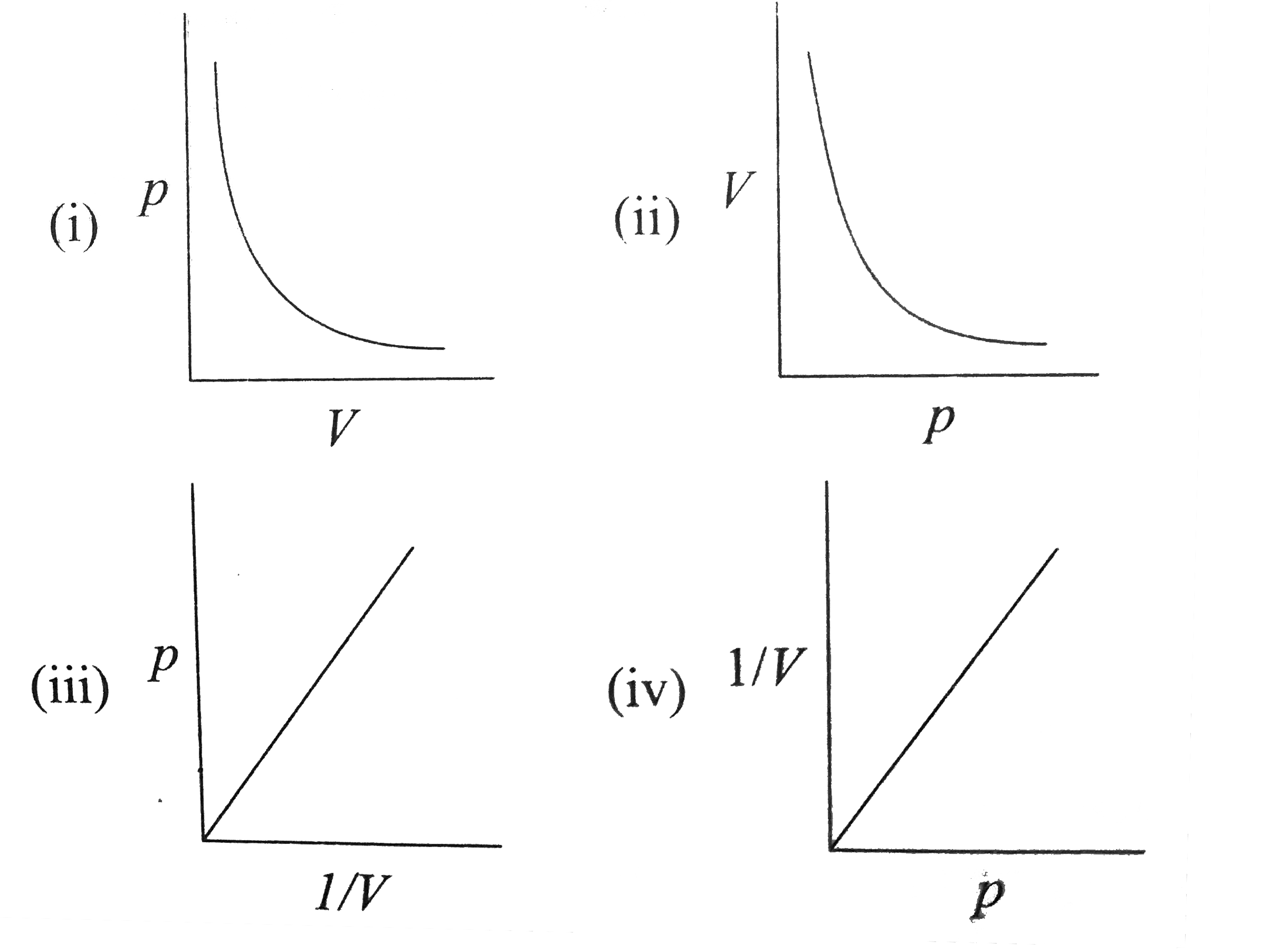
- Solved 2. (a) Derive an expression for the isothermal
- Physical Chemistry The Compression Factor (Z) [w/1 example

- Women's High Waist Yoga Pants Tummy Control Scrunched Booty

- Women Harem Pants/ Festival Pants/ Boho Clothing/ Hippie Pants

- Women's Knees Mesh Seamless High Waist Power Flex Yoga Pants Tummy

- Fun Cute Colorful Slouch Socks For Women Girls, Scrunch Socks Women, C – Happypop

- One Shoulder Chain Strap Cut Out Swimsuit | Karen Millen