Monday, Sept 16 2024
Solved The density of water is 1.00 g/mL at 48C. How many

By A Mystery Man Writer

Calculate the molarity of pure water (d=1/mL).

The specific heat capacity of liquid water is 4.18 kJ/g C, how

Solved The density of water is 1.00 g/mL at 4°C.How many
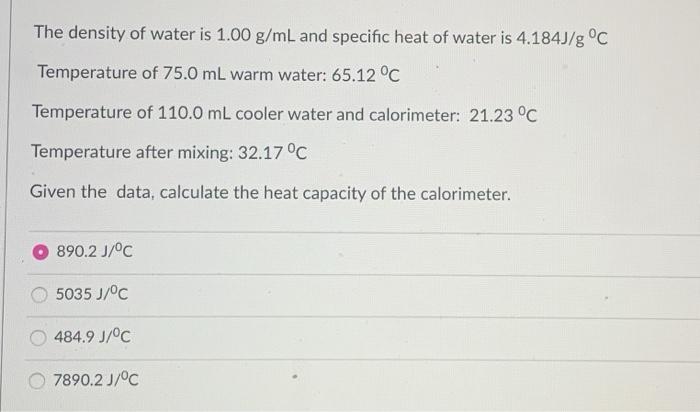
Solved The density of water is 1.00 g/mL and specific heat

Calculating Density Warm Up What is the density (g/cm3) of 48.0 g
What is the volume of a solution, in mL, of sucrose, (C12H22O11
✓ Solved: An aqueous solution is 1.00%NaCl by mass and has a
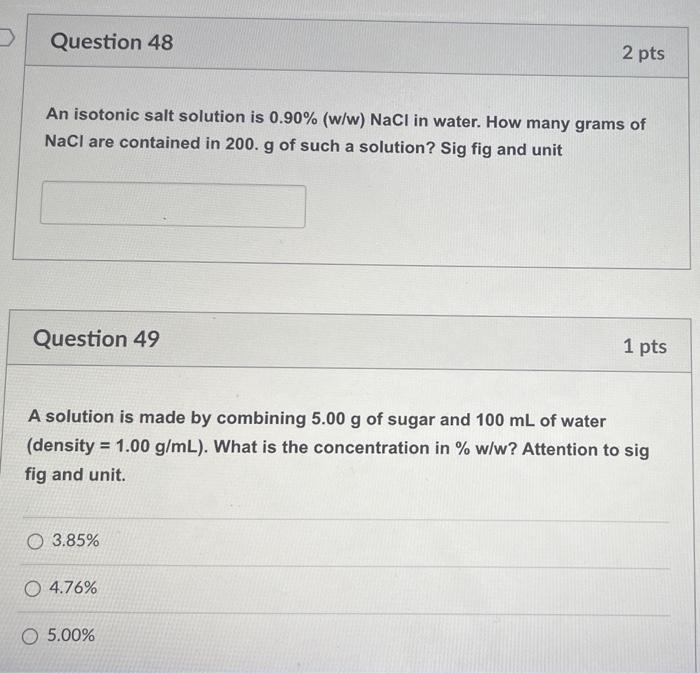
Solved Question 48 2 pts An isotonic salt solution is 0.90

Solved 2. Assuming that the density of water is 1.00 g/ml
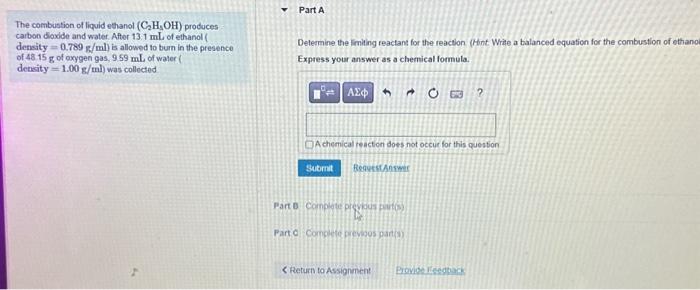
Solved The combustion of liquid ethanol (C2H3OH) produces
Related searches
- HP 48 series - Wikipedia

- Bakugan Series 4 - 17/48C Copper Gate Card - G-Power Swap

- Dryer temperature controller LC-48 injection molding machine temperature controller LC48 temperature adjustment - AliExpress

- Grass Roots by ESP G-LB-48C White Electric Bass Guitar

- Sold: G-Wind industries Clipper 48c One off Aluminium, Pre-owned

Related searches
- Salutation Stash Mesh 7/8 Tight

- jumpsuit women elegant pink

- Young trendz Panty For Girls Price in India - Buy Young trendz Panty For Girls online at

- Best Oatmeal with Yogurt and Toasted Almonds Recipe - How To Make Oatmeal with Yogurt and Toasted Almonds

- Trapeze Dress Sewing Pattern, Everyday Dress, Dress PDF Sewing

©2016-2024, travellemur.com, Inc. or its affiliates