The compressibility factor Z for an ideal gas will be

By A Mystery Man Writer
The compressibility factor Z for an ideal gas will be

68. The compressibility factor (z) an ideal gas is equal to which of the following values? (A) Zero (B) Less than one (C) Equal to one

This section contains 06 multiple choice questions. The answer to

Compressibility factor - Wikipedia

Compressibility factor Z = PV / nRT is plotted against pressure as shown below:What is the correct order for the liquefiability of the gases shown in the above graph? A. CO 2

The given graph represents the variation of Z (compressibility factor = \[\dfrac{{PV}}{{nRT}}\] ) versus P, for three real gases A, B and C. Identify the only incorrect statement.
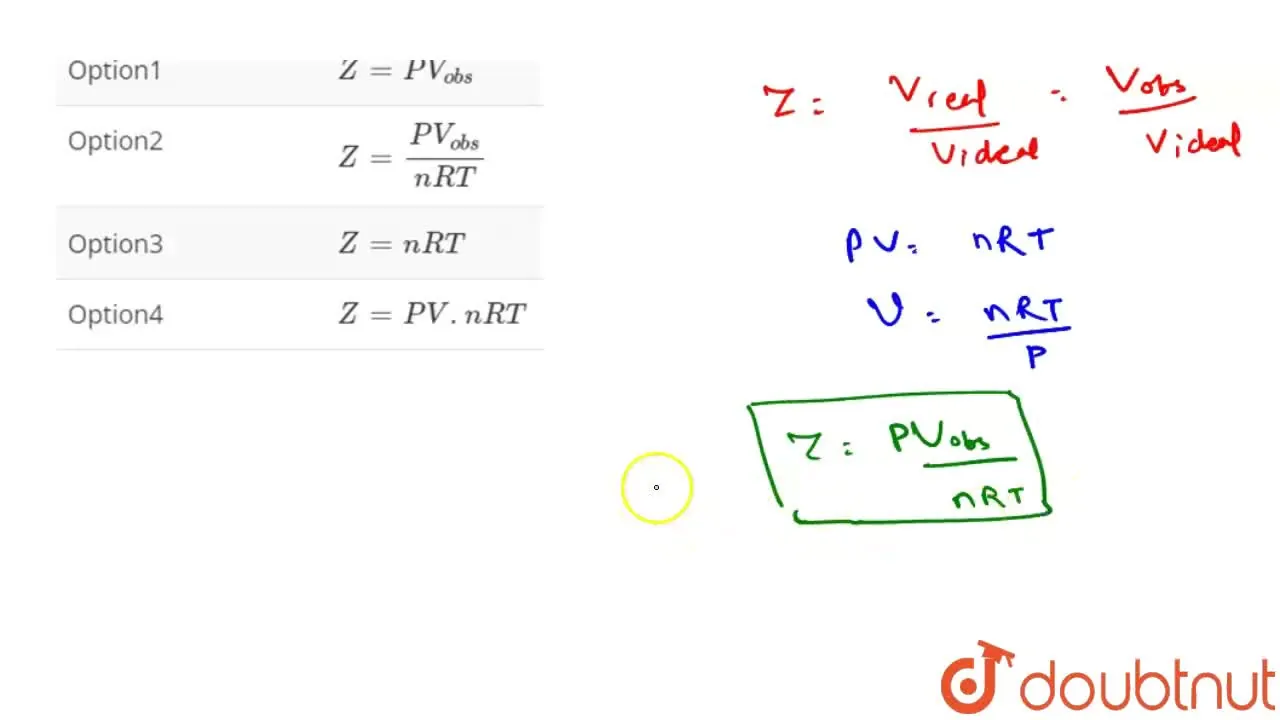
The compressibility factor Z for the gas is given by

Difference Between Real Gas and Ideal Gas, Compressibility Factor

Compressibility Factor of Gas, Overview, Equation & Chart - Lesson

4.2: Real Gases (Deviations From Ideal Behavior) - Chemistry LibreTexts

Question ( 13 quad ) QnDirections: The answer to the following

Mins section contains 5 questions. The answer is a single digit

Compressibility factor, Z of a gas is given as `Z=(pV)/(nRT)` (i) What is the value of Z for an
- Compressibility factor (gases) - Citizendium

- At low pressure, the van der waal's equation is written as (P+ a/V^2)V=RT . Then compressibility factor is then equal to
- Explain how the compression factor varies with pressure and

- PDF] COMPARISON OF FIVE NATURAL GAS EQUATIONS OF STATE USED FOR FLOW AND ENERGY MEASURMENT

- Procedure calculates base gas compressibility factors