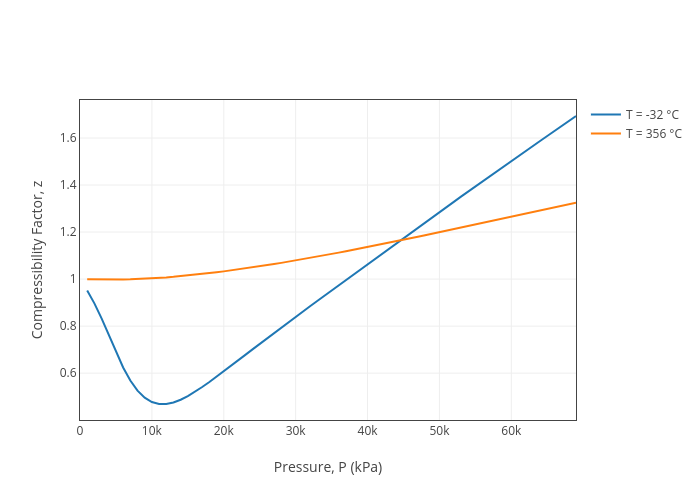
In the following compressibility factor (Z) vs. pressure graph 300 K, the compressibility of CH_{4} pressure < 200 bar deviates from ideal behaviour becauseThe molar volume of CH_{4} is than its molar

By A Mystery Man Writer
Click here:point_up_2:to get an answer to your question :writing_hand:in the following compressibility factor z vs pressure graph at 300 k the compressibility of
Click here👆to get an answer to your question ✍️ In the following compressibility factor -Z- vs- pressure graph 300 K- the compressibility of CH-4- pressure - 200 bar deviates from ideal behaviour becauseThe molar volume of CH-4- is than its molar volume in the ideal stateThe molar volume of CH-4- is than its molar volume in the ideal stateThe molar volume of CH-4- is same as that in its ideal stateIntermolecular interactions between CH-4- molecules decreases

Solved The graph of compressibility factor (Z)v/sP for 1 mol

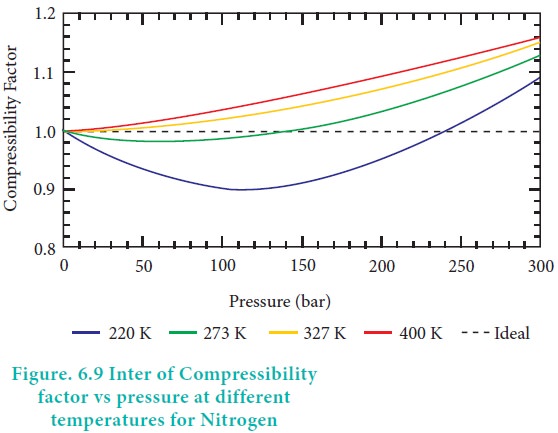
Reading Compressibility Factor Charts

WPILARIVIANN ZU 60. ollowing compressibility factor (2) vs pressure graph 300 K, the compresability of Cheatre 200 bar deviates from ideal behaviour because Compressibility Factor (2) Ideal gas 02 0 200 600

New explicit correlation for the compressibility factor of natural gas: linearized z-factor isotherms
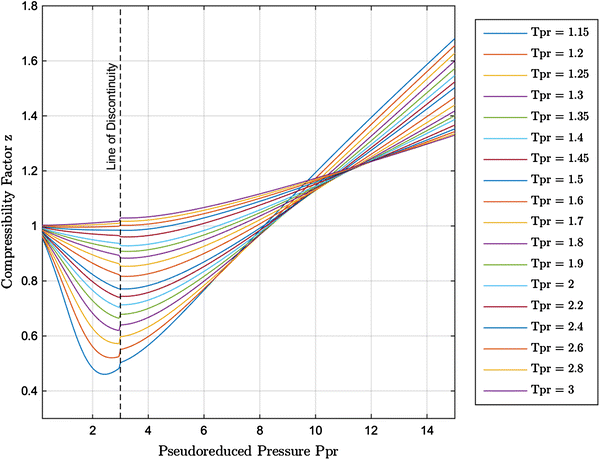
New explicit correlation for the compressibility factor of natural gas: linearized z-factor isotherms
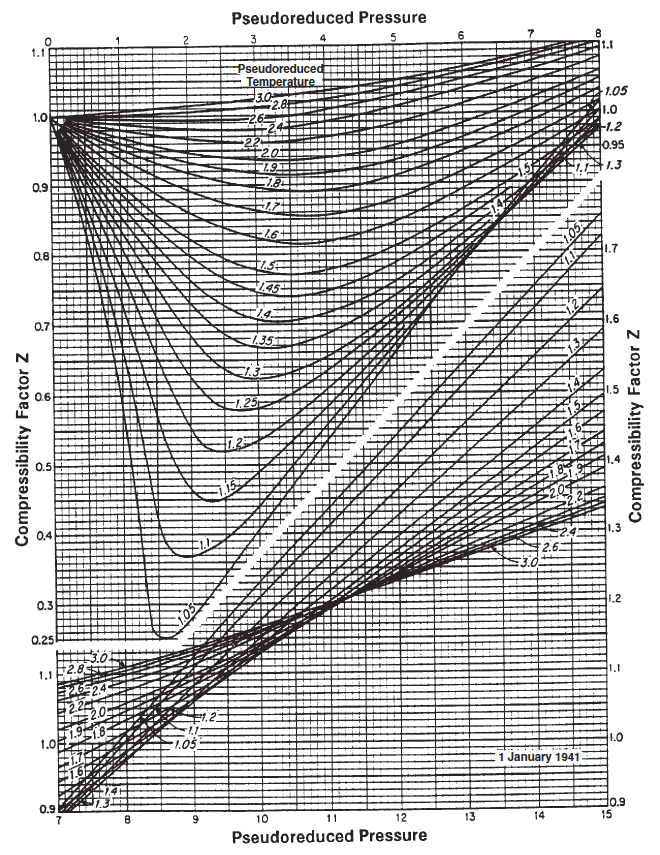
Real Gas Law - whitson wiki
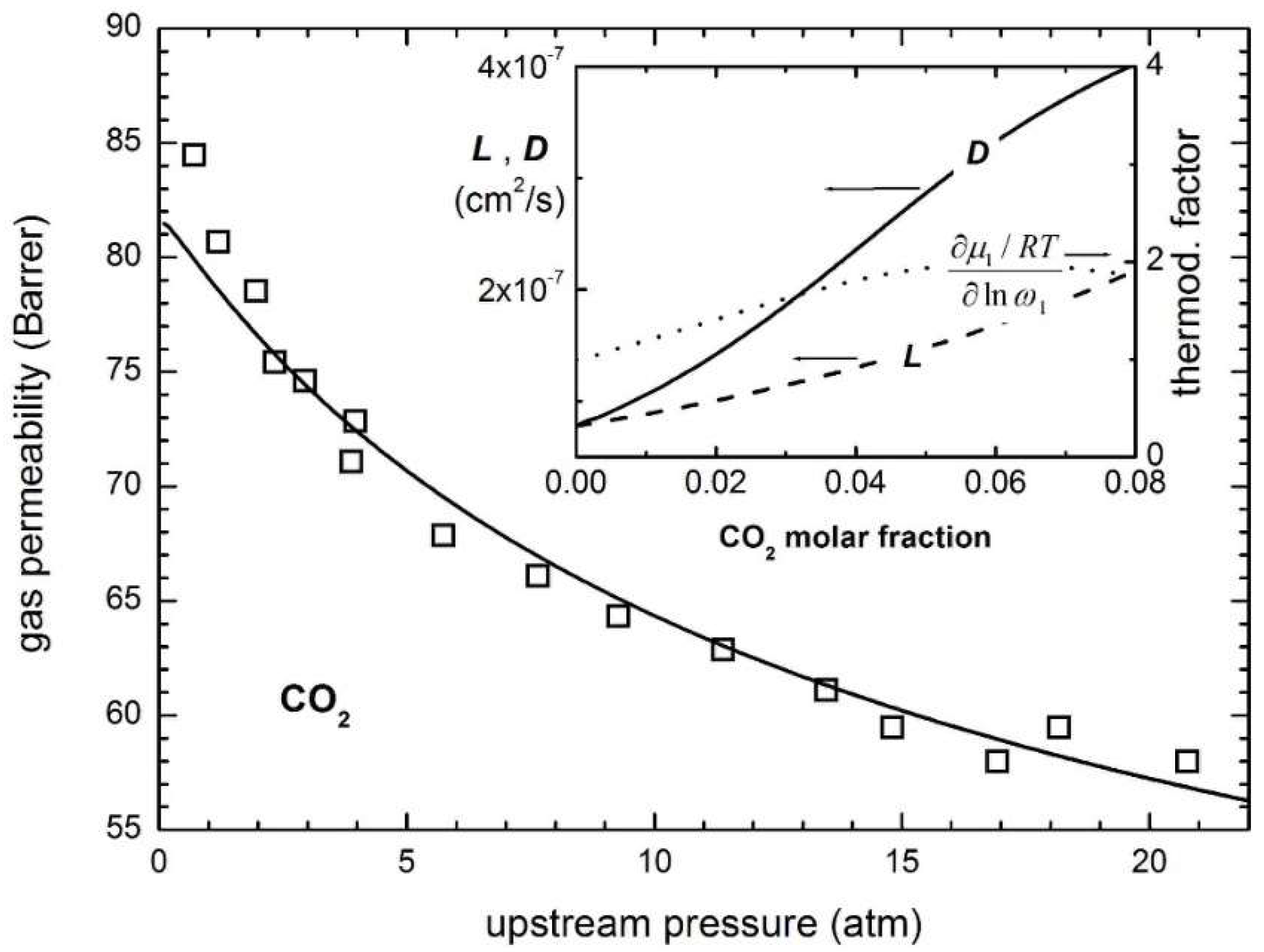
Membranes, Free Full-Text
Compressibility factor Z - Gaseous State

Why does ammonia deviate from ideal gas? - Quora

Solved QUESTION 3 Determine the compressibility

The graph of compressibility factor Z vs P for one mole of a real gas is shown in following diagram. The graph is plotted at a constant temperature 273 K. If the

Chem-Eng-Musings Ideas Concepts Formula's Data
- Essential Long Sleeve Crew Neck

- Bamboo Undergarment Fabric Women's Panties Girl Lingerie Ladies

- 6 Packs Curly Faux Locs Crochet Hair 18 Inch Goddess Locs Crochet Hair, Hippie Locs Braids, Boho Style Hair Extensions (18 Inch, 6Packs, TGray)

- TALA on LinkedIn: Best sustainable British activewear brands to know for 2023
- fvwitlyh Pants for Women Jean Pajama Pants Women Irregular Waist