Welcome to Chem Zipper.com: The compressibility factor for 1 mole of a van der Waals gas at 0oC and 100 atm pressure is found to be 0.5. Assuming that the volume of

By A Mystery Man Writer

At high pressure, the compressibility factor for one mole of van der w

The compressibility factor for a real gas at high pressure is

Kirkwood–Buff-Derived Force Field for Peptides and Proteins: Applications of KBFF20
The compression factor (compressibility factor) for one mole of a van der Waals' gas at 0ºC and 100 atm pressure is - Sarthaks eConnect

1148 questions with answers in GAS

Se PDF, PDF, Stress (Mechanics)

What is the compressibility factor Z for 0.02 mole of a van der waal's gas at pressure of 0.1 atm. Assume the size of gas molecule is negligible. Given: RT =20 L

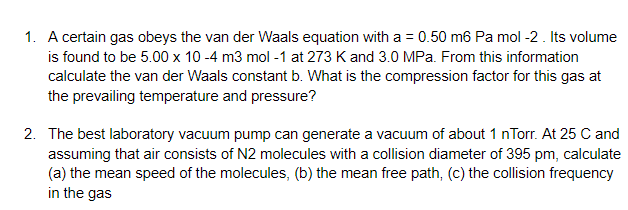
Solved A certain gas obeys the van der Waals equation with a

I need help with question 3: a,b,c, i'm stuck and

Solved APPENDIX Problem 1: Molar Volume and Compressibility
- Gas Compressibility Factor Calculator Excel SpreadsheetLow Cost Easy to Use Spreadsheets for Engineering Calculations Available at Engineering Excel Spreadsheets

- Class Notes on Compressibility of a Real Gas, CH 417, Study notes Physical Chemistry

- Procedure calculates base gas compressibility factors
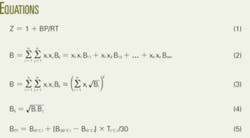
- Developing a Thermodynamical Method for Prediction of Activity

- The compression factor (compressibility factor) for one mole of a van der Waals' gas - Sarthaks eConnect




