Indian industry group calls for clearer expiry date labelling rules for topic drugs

By A Mystery Man Writer
The DCGI needs to clarify expiry date labelling rules for topical drugs according to an Indian trade group, which says some members’ products are being deemed to be ‘misbranded.’

Labeling Guidelines for Cosmetics as per the Drugs & Cosmetics Act - NKG Advisory Business & Consulting Services Pvt. Ltd

Labelling and Packaging for Drugs: Ensuring Safety and Compliance - Asian Community News

Cold Calling Scripts: 25 Script Examples & Call Tips

French regulator raises concerns over Indian CRO studies

China e-cigarette titan behind 'Elf Bar' floods the US with illegal vapes

17 առօրյա իր, որոնց պիտանելիության ժամկետի մասին մենք նույնիսկ գլխի չենք ընկնում (լուսանկարներ)

Food Laws And Regulations In India - Corpseed

PDF) Drug labeling: The study of compliance of regulatory requirements for prescription drugs in India

Has this food actually expired? Why label dates don't mean what you think, Food waste
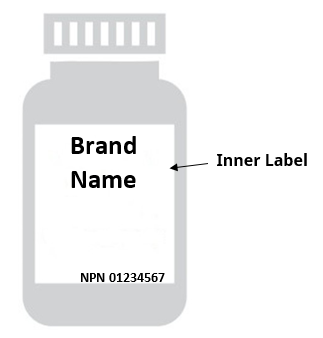
Guidance document: Labelling of natural health products

The Difference Between Best Before and Expiry Dates

Labeling in india

Cold chain - Pharma

Labeling Requirements Registration of Medical Devices India

The Good Life, Book by Robert Waldinger, Marc Schulz, Official Publisher Page
:max_bytes(150000):strip_icc()/expiration-food-4595d0ae170a422fbf7cd2e87740b910.jpg)








