An ideal gas initially P_i ,V_i , and T_i is taken through a cycle

By A Mystery Man Writer
Click here:point_up_2:to get an answer to your question :writing_hand:an ideal gas initially at pi vi and ti is taken through a cycle
Click here👆to get an answer to your question ✍️ An ideal gas initially P-i -V-i - and T-i is taken through a cycle as shown in Figure- -a- Find the net work done on the gas per cycle 1-00 mol of gas initially 0-0C- -b- What is the net energy added by heat to the gas per cycle

Thermodynamics

An ideal gas initially P_i ,V_i , and T_i is taken through a cycle as shown in Figure. (a) Find the net work done on the gas per cycle 1.00 mol of
Solved] An ideal gas described by Ti = 275 K, Pi = 1.10 bar, and Vi = 10.0

One mole of an ideal gas is contained in a cylinder with a movable piston. The initial pressure, volume,and temperature are P_i, V_i, and T_i, respectively. Find the work done on the

An ideal gas is taken through the cycle `AtoBtoCtoA,` as shown in the figure, If the net heat

Nanoreactors in action for a durable microactuator using spontaneous combustion of gases in nanobubbles

1st law

Process integration, energy and exergy analyses of a novel integrated system for cogeneration of liquid ammonia and power using liquefied natural gas regasification, CO2 capture unit and solar dish collectors - ScienceDirect

A 1.00 mol sample of monoatomic ideal gas is take through the cycle shown. At point A, the pressure, volume and temperature are P_i, V_i and T_i respectively. In terms of R
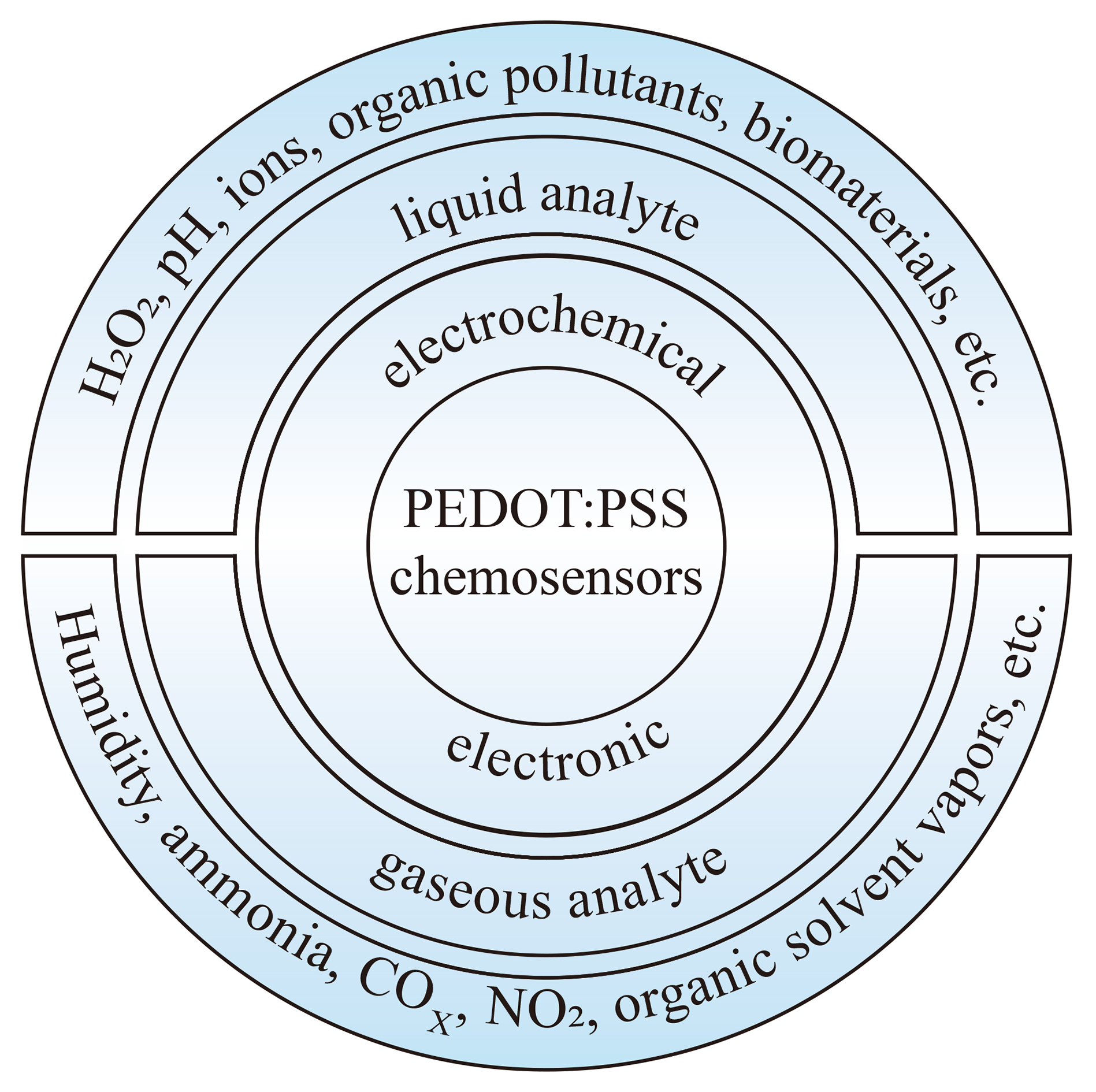
Chemosensors, Free Full-Text

D., Q
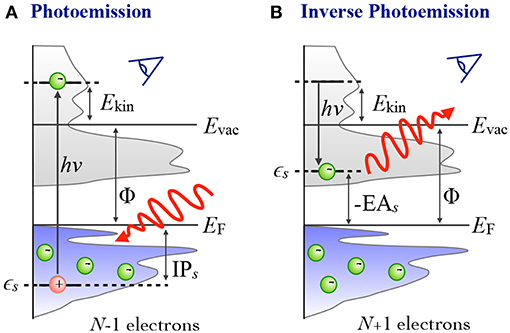
Frontiers The GW Compendium: A Practical Guide to Theoretical Photoemission Spectroscopy
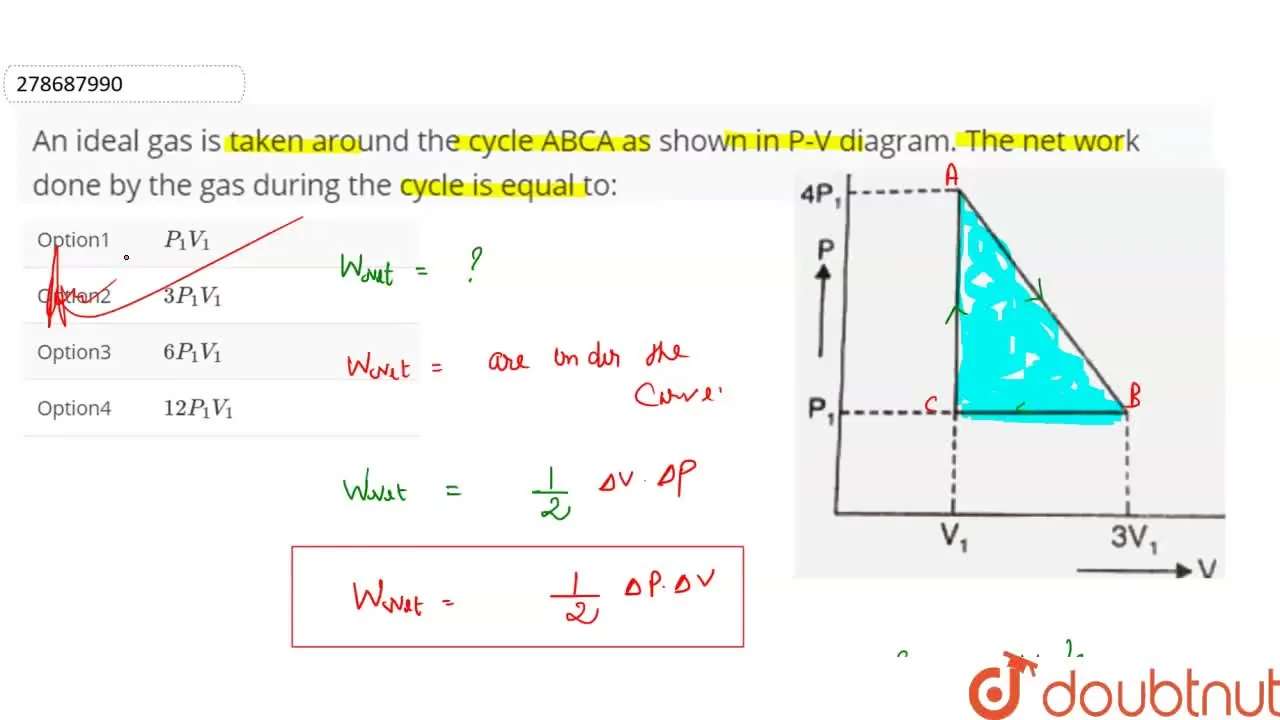
An ideal gas is taken around the cycle ABCA as shown in P-V diagram. T
- What Is the Best Way to Get Rid of Poison Ivy?
:max_bytes(150000):strip_icc()/what-is-the-best-way-to-get-rid-of-poison-ivy-4864234-07-bee27156f690470482c70af7c5b2ce7f.jpg)
- Auden Womens Padded Ribbed Bralette Bra Size XS Pink/Lavender

- Women'S Seamless Waist Trainer Cincher Corset Breathable Invisible Body Shaper Waist Training Tummy Control

- Juicy Couture - pink bra, size 38D, NWOT - $30 (34% Off Retail) - From Ashly

- Spanx Undie-Tectable Thong - Soft Nude