If Z is a compressibility factor, van der Waals' equation at low pressure can be written as - Sarthaks eConnect
By A Mystery Man Writer
If Z is a compressibility factor, van der Waals

If Z is a compressibility factor, van der Waals' equation at low press
The compression factor (compressibility factor) for one mole of a van der Waals' gas - Sarthaks eConnect

Compressibility factor (gases) - Citizendium
The value of compression factor at the critical state of a vander waals gas is
If Z is a compressibility factor, van der Waals equation at low pressure ..

Radical Occupation, Radical Spatiality. Unconference. Think Space., PDF
20.If Z is a compressibility factor, van der Waals equation at low pressure can be written as

If Z is a compressibility factor, van der Waal's equation low pressure can be written as : tot gnolaszemit sem st263 nisho ad Phim shuplamenu Pb (1) Z = 1 - (
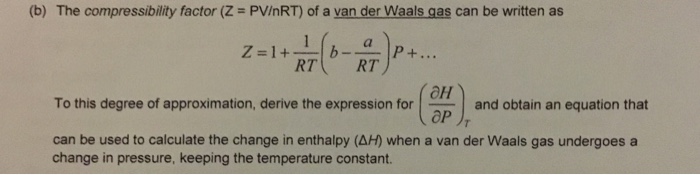
Solved (b) The compressibility factor (Z - PV/nRT) of a van

At low pressures For 1 mole, the van der Waals equation is written as [ p + a / V 2] V = RT The compressibility factor is then equal to:A. 1
- The value of compression factor at the critical state of a vander waals gas is
- Real gasses For an ideal gas, the compressibility factor Z = PV/nRT is equal to unity for all conditions. For a real gas, Z can be expressed as a function. - ppt

- Thermodynamics - 3-7 Ideal Gas Equation with compressibility

- Non Ideal Gas Behavior-chemistry - Non Ideal Gas Behavior

- Compressibility Factor Z for sub-critical pressures for Lee





