Solved) - For values of z near 1, it is a good approximation to write z(P) = - (1 Answer)
By A Mystery Man Writer
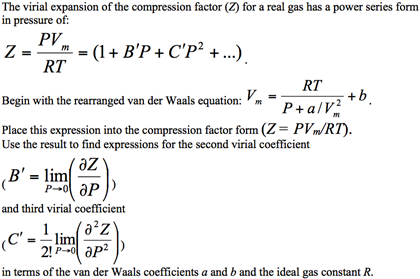
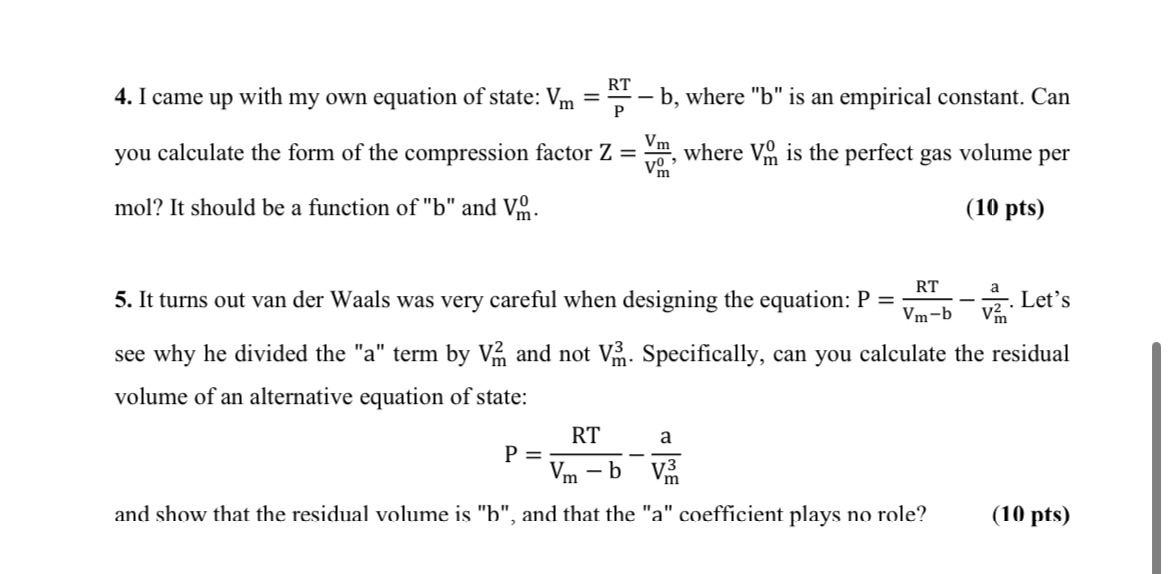
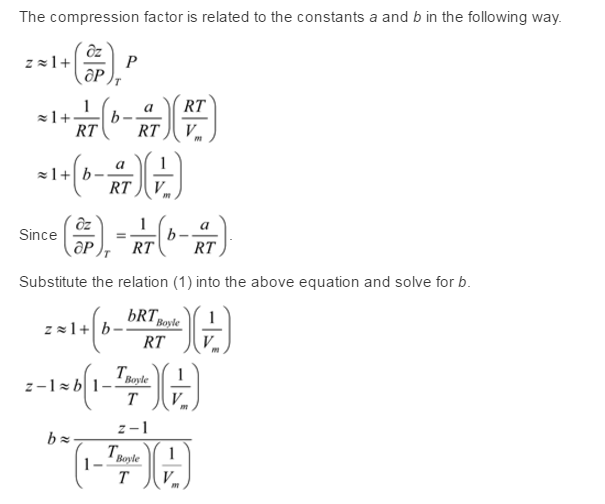
For values of z near 1, it is a good approximation to write z(P) = 1 + ( z/ P) T P if z = 1.00104  at 298 K and 1 bar, and the Boyle temperature of the gas is 155 K, calculate the values of a, b, and  for the van der Waals gas.  

Riemann zeta function - Wikipedia
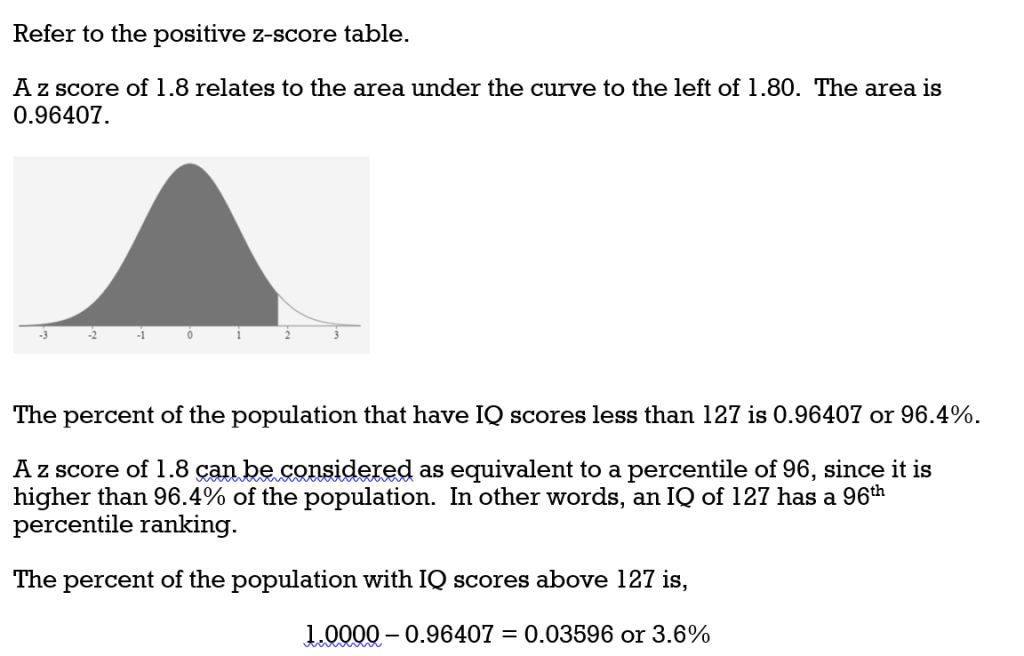
8.4 Z-Scores and the Normal Curve – Business/Technical Mathematics

SOLUTION: Normal Approximation To Binomial - Studypool
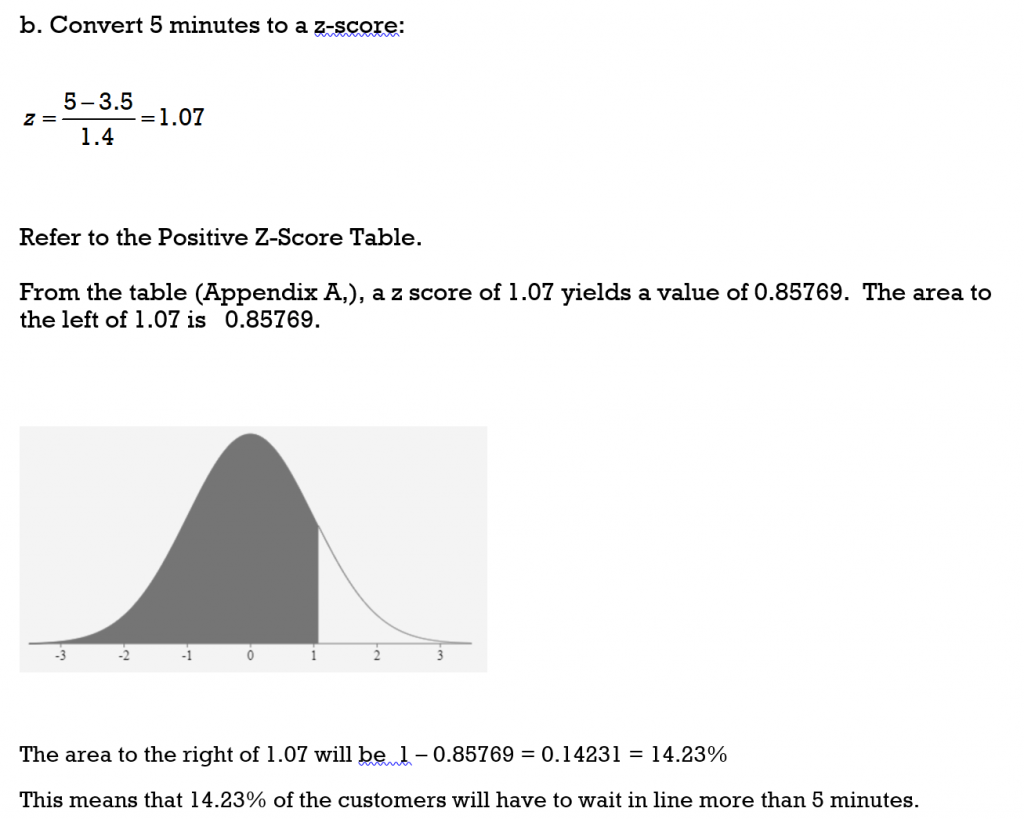
8.4 Z-Scores and the Normal Curve – Business/Technical Mathematics
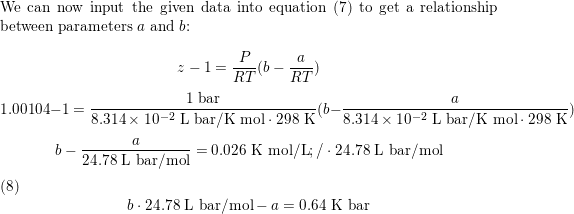
For values of $z$ near 1, it is a good approximation to writ
:max_bytes(150000):strip_icc()/LeastSquaresMethod-4eec23c588ce45ec9a771f1ce3abaf7f.jpg)
Least Squares Method: What It Means, How to Use It, With Examples

Solved Find the center of mass of the solid cone bounded by
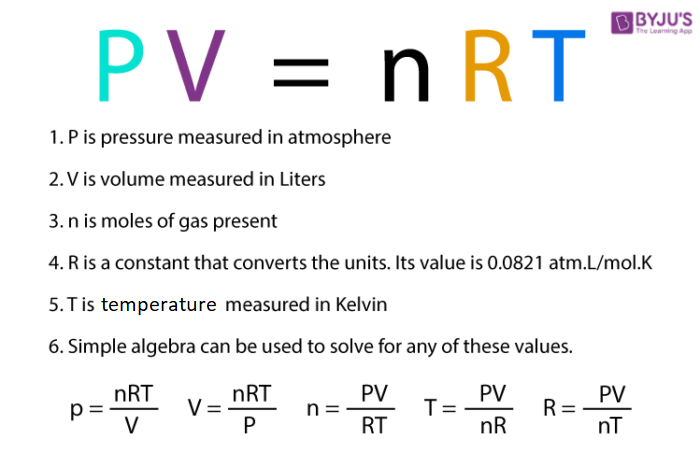
Ideal Gas Law Equation Compressibility Of Natural Gas - Chemistry

6.2 Using the Normal Distribution