FDA has new mammogram guidelines for dense breast disclosure. What
/cloudfront-us-east-1.images.arcpublishing.com/pmn/EMEDJI4AQVBZJNJHKLTY2ZSPJY.jpg)
By A Mystery Man Writer
In Pennsylvania, senators unanimously voted in favor of a bill to fund genetic testing to women at higher risk of breast cancer.

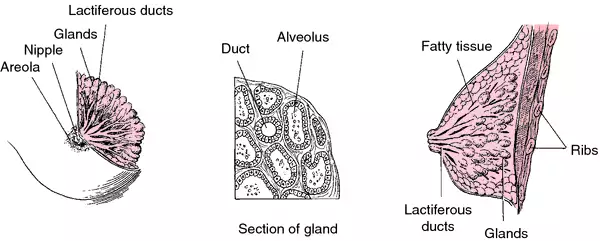
Breast Density and Breast Cancer Risk: A Practical Review

U.S. to require breast density information after mammograms
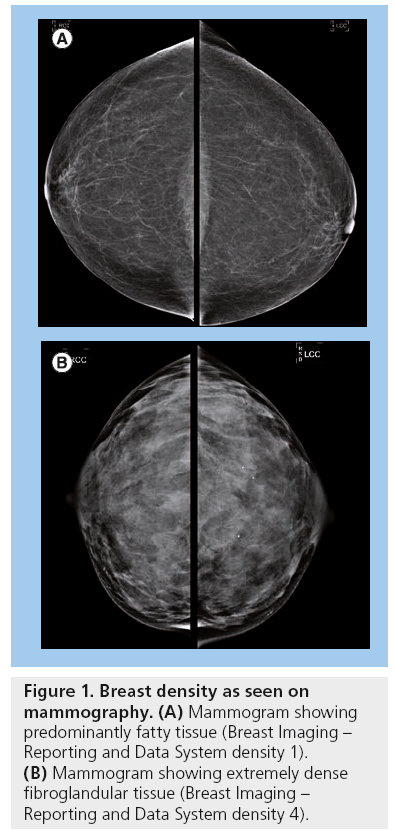
Automated breast ultrasound: a novel approach to screening women

Automated breast ultrasound: a novel approach to screening women

FDA mammography update raises cost questions

New breast cancer screen FDA mandate will begin in September 2024
FDA to require mammogram providers to notify women about breast

FDA Will Require Dense Breast Disclosure at Mammogram Clinics
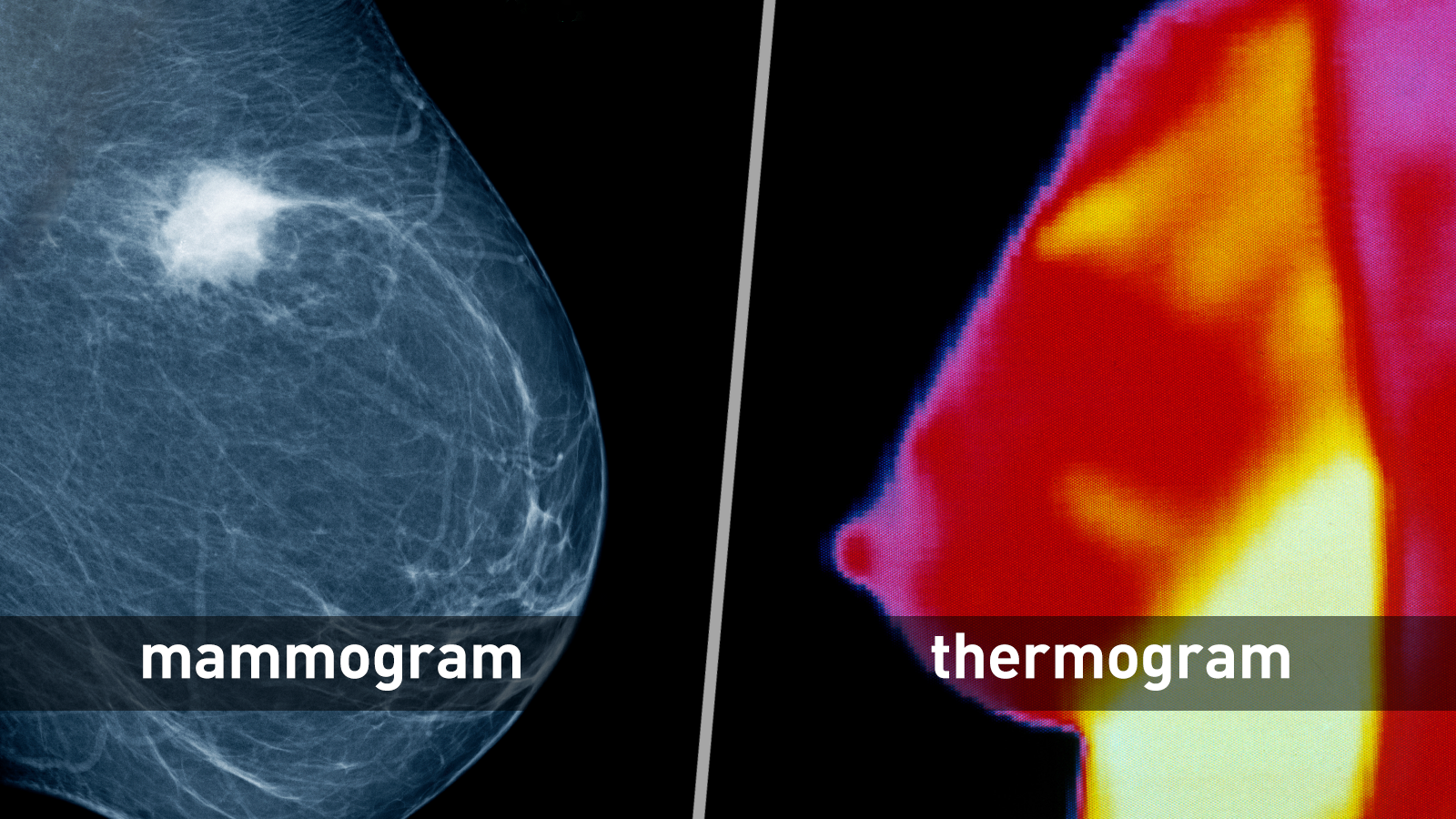
Breast Cancer Screening: Thermogram No Substitute for Mammogram

image.slidesharecdn.com/acogsnowmeetingstalburg2-5

FDA dense breast rule promotes earlier cancer detection

Rad Tech CE, ASRT, ARRT® CE, Category A Credits
Breast Cancer Screening (PDQ®) - NCI
- Reebok Women's Prime Essential Medium Impact Sports Bra Back

- Swarovski no Brasil

- Thermal nderwear for Women Fleece Lined Thermals Women's Base Layer Long John Set

- Vestidos de tirantes con flecos y borlas para mujer, ropa de baile a la moda, con accesorios de los años 20

- Keep It Live (studio album) by Dazz Band : Best Ever Albums
:max_bytes(150000):strip_icc()/why-red-blotches-and-itchy-breasts-may-mean-cancer-513594_final-85bf193d4ac54df1b483f3f762300e07.png)