Preparation of Standard Solution of Sodium Carbonate - Chemistry

By A Mystery Man Writer
A common primary standard for standardizing strong acids is sodium carbonate (Na2CO3).For acid-base titration, it is customary to prepare solutions of an acid and base of the desired concentration. Visit BYJU
A common primary standard for standardizing strong acids is sodium carbonate (Na2CO3).For acid-base titration, it is customary to prepare solutions of an acid and base of the desired concentration. Visit BYJU'S to understand more about it.
Acids-Bases and Salts-Volumetric analysis, Chemistry tutorial

Title: Lesson 14 Preparing a Standard Solution and Back Titration

Titrating sodium hydroxide with hydrochloric acid

Give two important uses of washing soda and baking soda - Teachoo
Sodium carbonate - Wikipedia

Question Video: Determining the Concentration of Sulfuric Acid Via

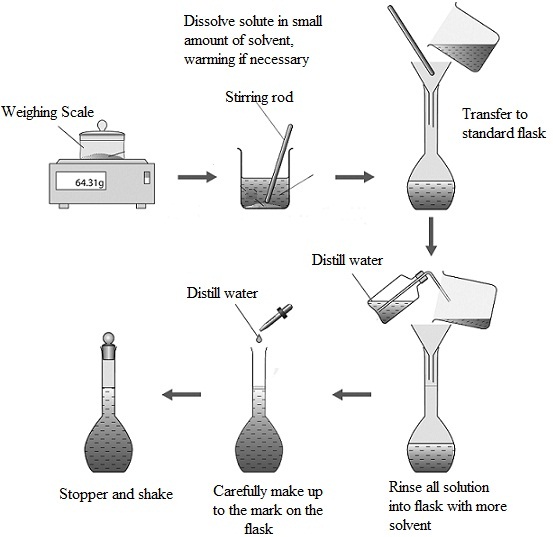
Preparing a standard solution - sodium carbonate

Chemistry Lab Report: Preparation Of Solution

SOLUTION: Preparation of Sodium Carbonate by Solvay's Process
- Circle Skirt Formulas: Calculate Your Circle Skirt Radius! Circle skirt, Circle skirt pattern diy, Circle skirt pattern

- What size am i calculator - The Tech Edvocate

- What Size Am I Women's Calculator - Cuddl

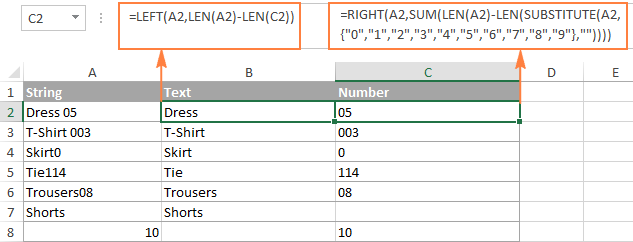
- Excel: Split string by delimiter or pattern, separate text and numbers
- Cool Funny Calculator Party and Halloween Costume Design Graphic T-Shirt Dress for Sale by The1Tee

- Cinturilla colombiana 24/7 - Comprar en Mundo Fit by Ls
- Top 21 Best Pilates classes near Montreal, Canada Updated March 2024
- Checked Tapered Ankle Grazer Trousers
- Sweatshirt Blazer Women Solid Casual Open Front Womens Lightweight Jacket Long Sleeve Slimming Personalized formal

- The Sydney bra is the only thing I workout in now. A blend to run, lift and lounge in. ⠀⠀⠀⠀⠀⠀⠀⠀⠀⠀⠀ Softer than a binder yet more flattening…


