The given graph represent the variations of Z Compressibility factor Z PV nRT versus p for three real gases A B and C Identify the only incorrect statement

By A Mystery Man Writer

The given graph represents the variations of compressibility factor `Z=PV// nRT` vs `

Compressibility factor - Wikipedia

Yucation The given graph represent the variations of Z (compressibility factor = pV) v/s p three nRT real gases, A, B and C. Identify the incorrect statement. p(atm) - A. For the
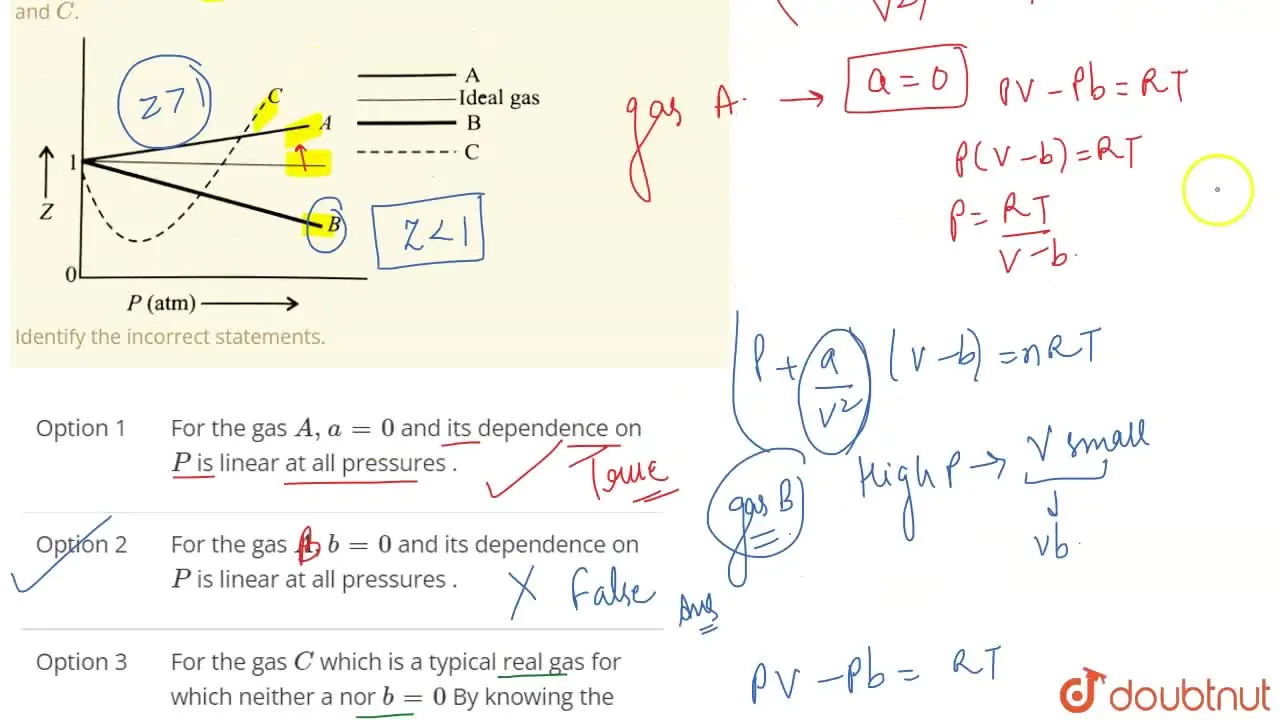
For the gas C which is a typical real gas for which neither a nor b =0

Compressibility factor, Z of a gas is given as Z=(pV)/(nRT) (i) What
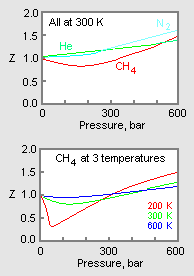
Compressibility factor (gases) - Knowino

From the given plot between Z and P , which of the following statements are correct for a real gas?

physical chemistry - Pressure vs volume plot for real gas and ideal gas - Chemistry Stack Exchange

variations of 2 12.7 (a) eb (c)-(ar (d) - 6. The given graph represent the variations (compressibility factor (Z)=- gases A, B and C. Identify the only incorrect statement pl) versus p

Non-Ideal Gas Behavior Chemistry: Atoms First
- At Critical Temperature,pressure and volume . The compressibility Factor (Z) Is
- Real Gases vs Ideal Gases & the Compressibility Factor
- Determine Compressibility Factor with Present of CO2 and H2S
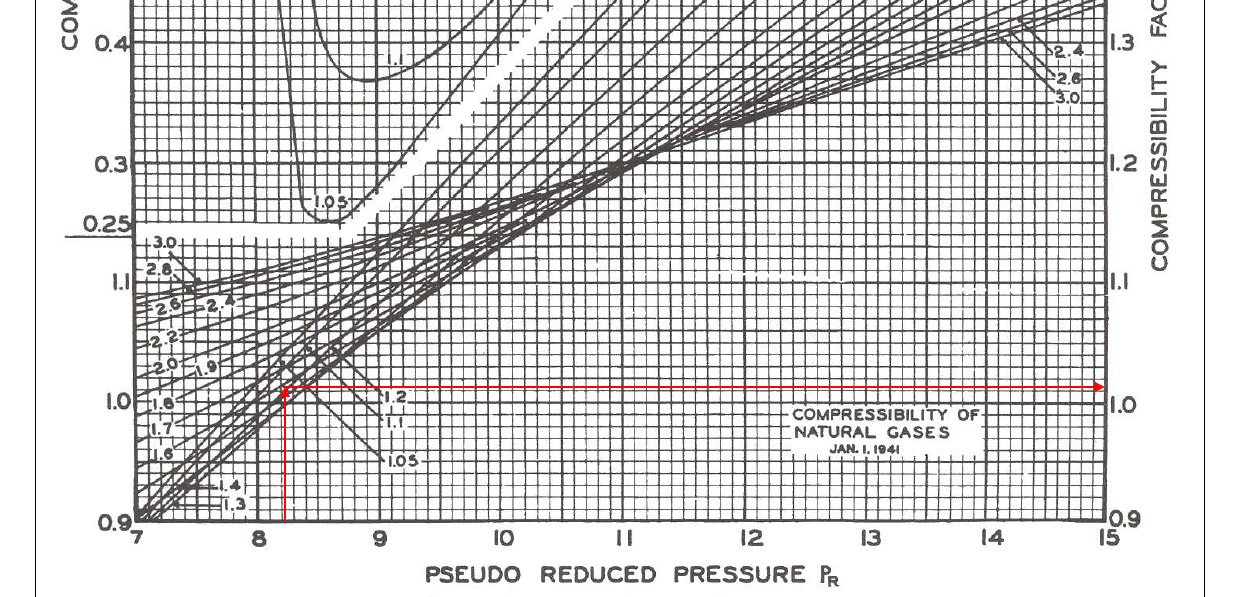
- The role of the compressibility factor Z in describing the volumetric behavior of gases

- plotting - How to plot Compressibility factor Z vs Pressure P using ParametricPlot? - Mathematica Stack Exchange

- Shop Purple Brand Jeans available now in stores + online
- No643 My Brazil minimal movie poster T-Shirt by Chungkong Art - Fine Art America

- Buy PSD Underwear Women's Underwear Food Boy Short

- Strawberry Fields Top - Streetwear Society Aesthetic Clothes

- The 10 Biggest TikTok Fashion and Beauty Trends of 2020


