total number of atoms in 44 g of Carbon dioxide is ?
By A Mystery Man Writer
Total number of atoms in 44 g of Carbon dioxide is
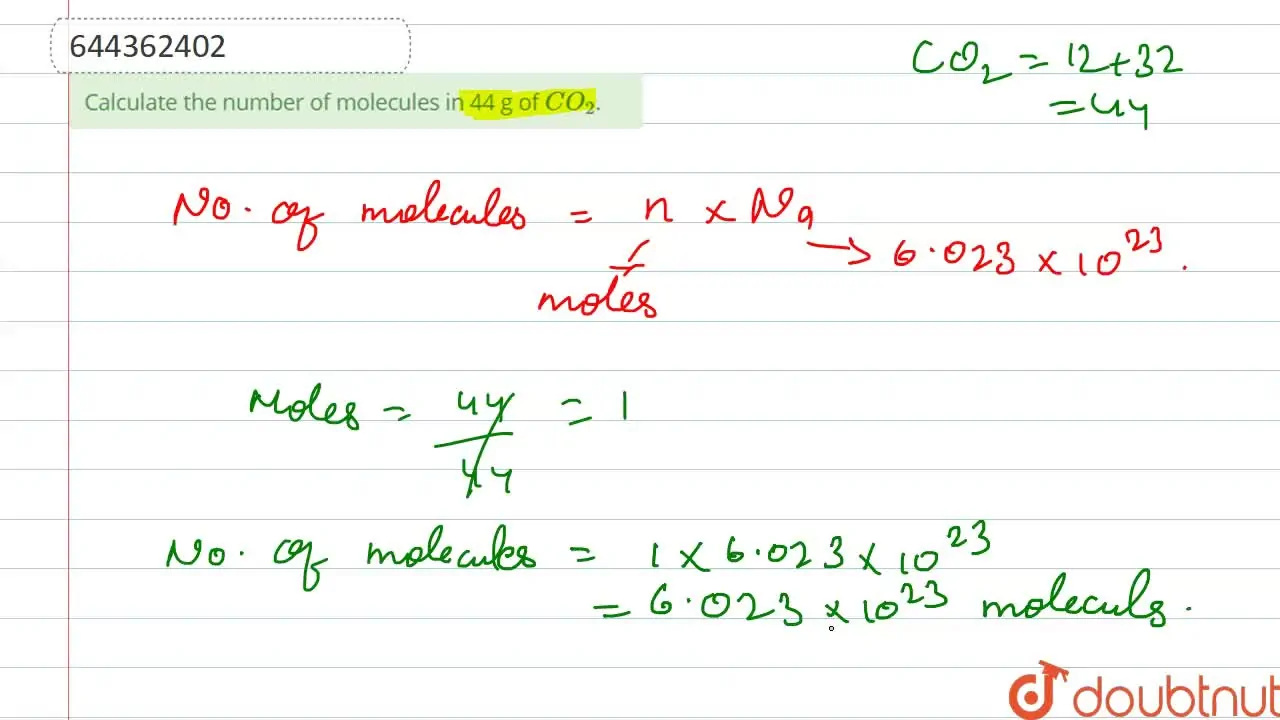
Calculate the number of molecules in 44 g of CO2.
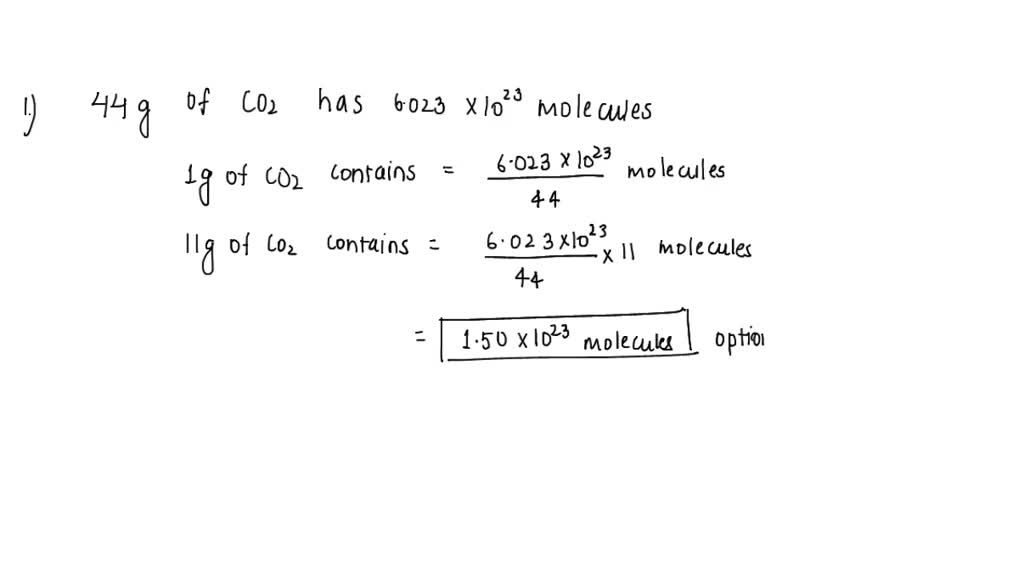
SOLVED: A 11.0 g sample of carbon dioxide (molar mass 44.0 g/mol). How many molecules does it contain? A. 6.02x10^23 B. 3.01x10^23 C. 1.50x10^23 D. 2.40x10^24 2. What is the mass of

How many atoms are present in 44 gm of CO2? - Quora

Which has the maximum number of molecules among the following?(a) 44 g CO2 (b) 48 g O3(c) 8 g H2

The Mole A very large counting number = - ppt download

What is the total number of atoms in 44G of CO2? - Quora
An Overview of Enabling Catalysts for Carbon Dioxide Conversion Aiming at the Two-carbon Target - Aerosol and Air Quality Research

Solved 1) How many O atoms are in 4.39 g of CO2? Molar Mass

SOLVED: calculate the total number of atoms present in 44 gram of carbon dioxide

Solved Calculate the mass of carbon dioxide (CO2, molar mass
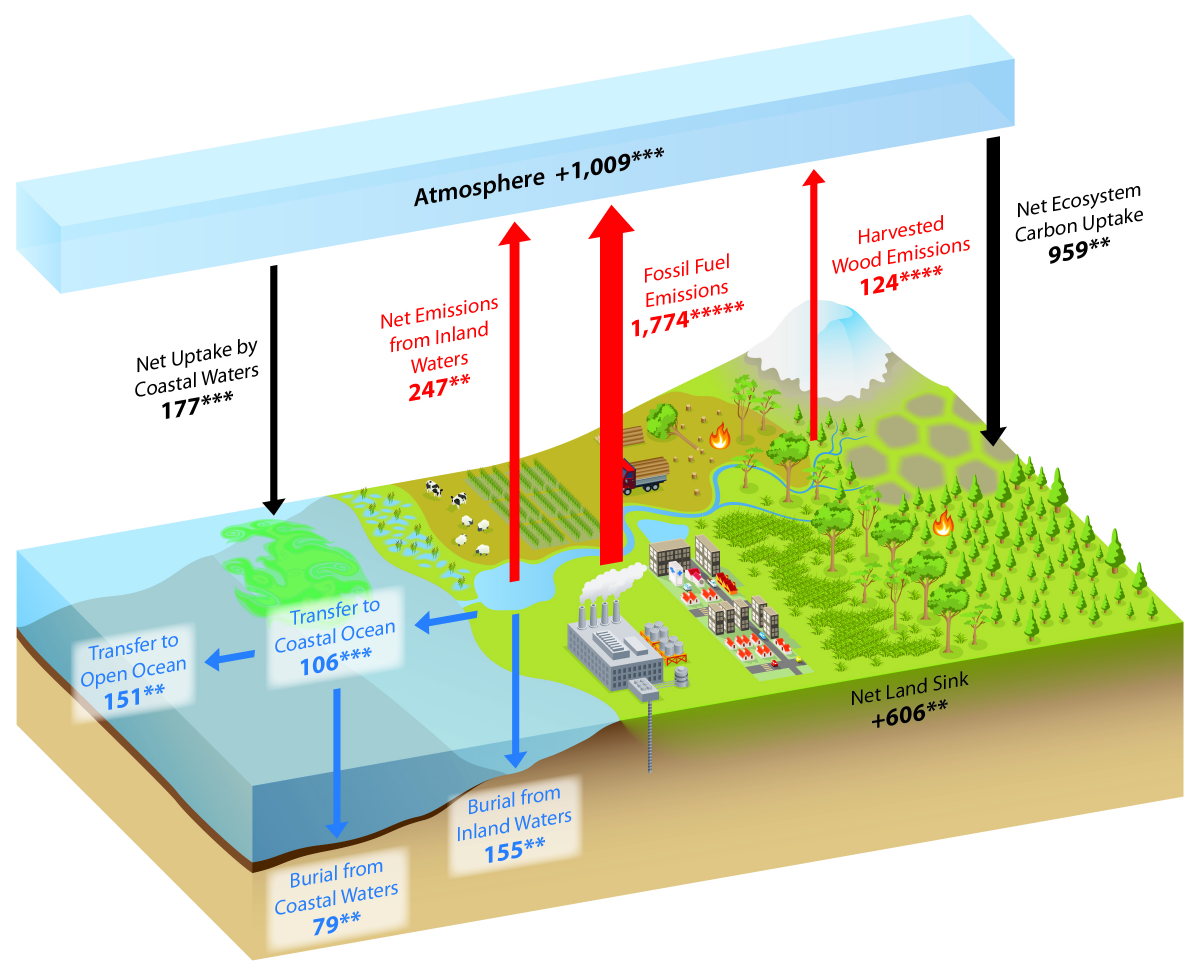
What is the Carbon Cycle? What is the science behind it?
- 2023 Toyota Tundra with 22x12 -44 G-FX Tm6 and 305/45R22 General Grabber Uhp and Leveling Kit

- Pyrodex® 44-45 Caliber/30 Grain Pistol Pellets

- G) FORD F650 EXECUTIVE VIP SHUTTLE BUS (up to 44 passenger)(No Luggage) - A&A Limousine & Bus Service

- Kit 6 pacote bombom ao leite recheio brigadeiro 4 un. 44 G em Promoção na Americanas

- Sandwich Neo Choco - Gelatelli - 4 x 44 g (176 g)

- PEESAFE Natural Anti Chafing Stick | Chub Rub Stick | Anti Chafe Balm | Anti Sweat Stick | Anti Friction Stick | Anti Blister Balm | Anti Chafing

- Buy RBX women 2 pack brand logo padded sports bra gray black
- Fall 2024 Ready-to-Wear Trend: Tailoring [PHOTOS]

- CRZ YOGA Women's Size Medium (8/10) Black 25 Drawstring Pockets

- Beautiful Woman Body In Shape. Loseup Healthy Girl With Fit Slim
