a) The original value of the reaction quotient, Qc, for the

By A Mystery Man Writer
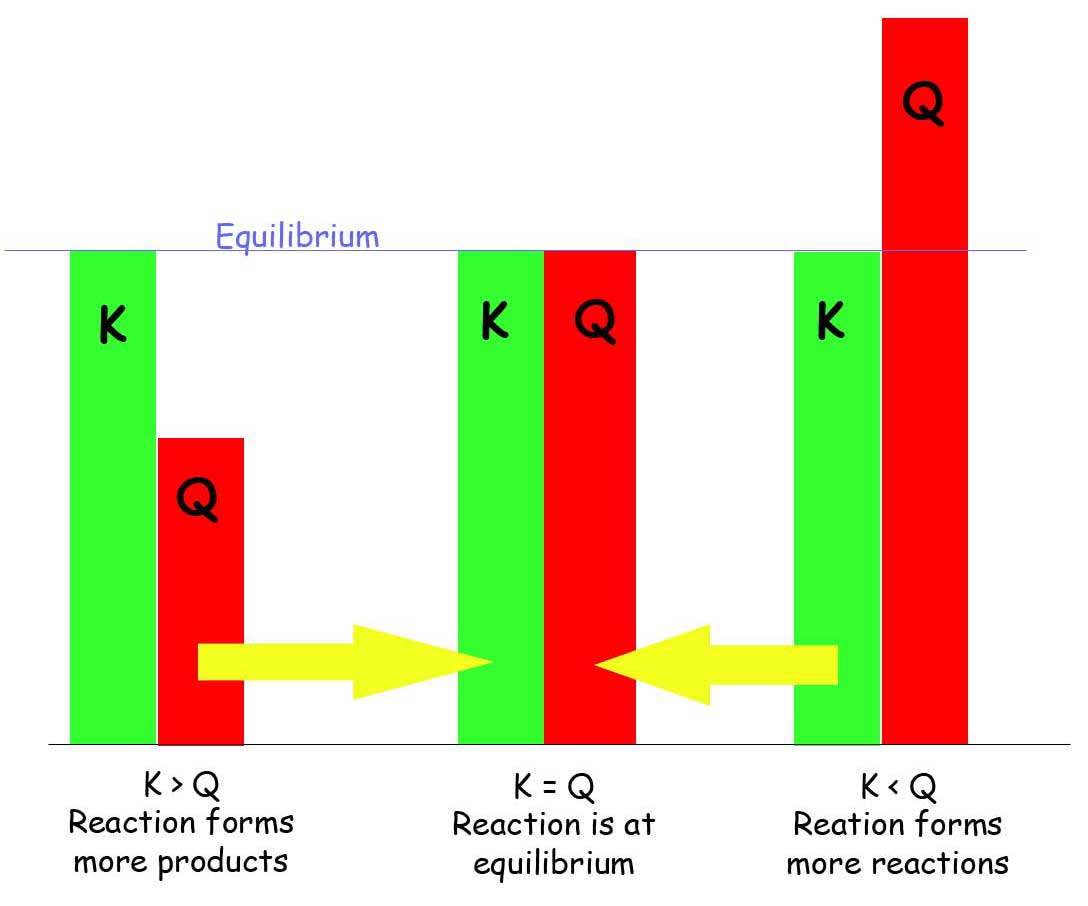
Gas Equilibrium Constants - Chemistry LibreTexts

Review for Exam 1 Chem 173

SOLVED: The reaction quotient, Qc for a reaction has a value of 75 while the equilibrium constant; Kc has a value of 195. Which of the following statements is accurate? Select one
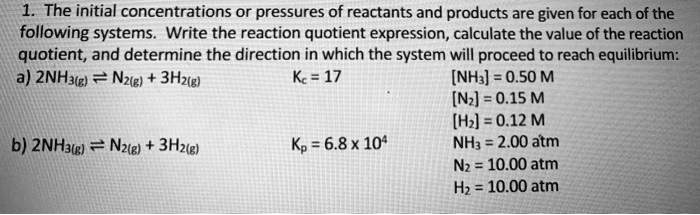
SOLVED: The initial concentrations or pressures of reactants and products are given for each of the following systems. Write the reaction quotient expression, calculate the value of the reaction quotient, and determine

Unit 7 FRQ.docx - Unit 7 FRQ For parts of the free-response
Answered: 0.080- 0.070 0.060- 0.050- 0.040-…
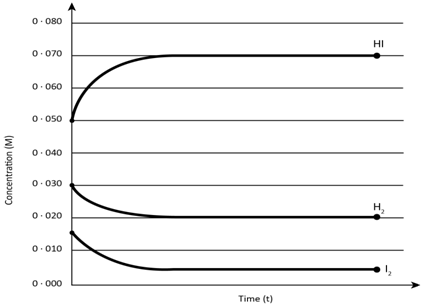
Answered: 0.080 0.070 0.060 0.050 0.040 0.030…

Unit 5 FRQ: Progress Check KEY (pdf) - CliffsNotes
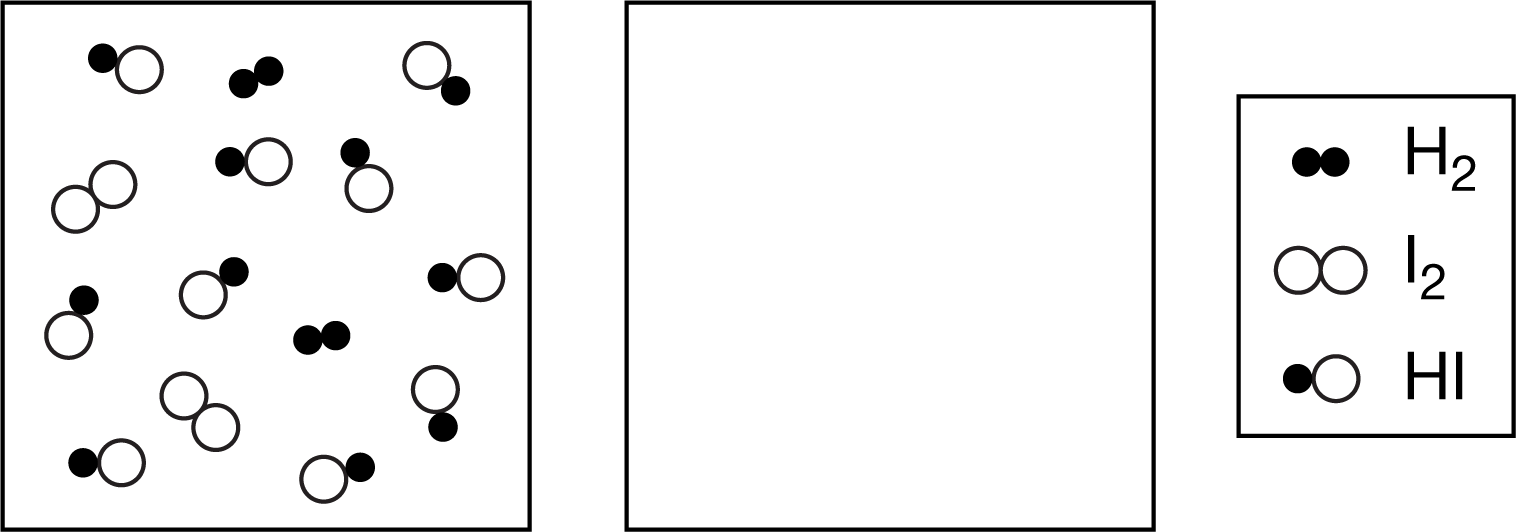
Answered: 0.080- 0.070 0.060- 0.050- 0.040-…

Equilibrium Constant and Reaction Quotient - MCAT Physical
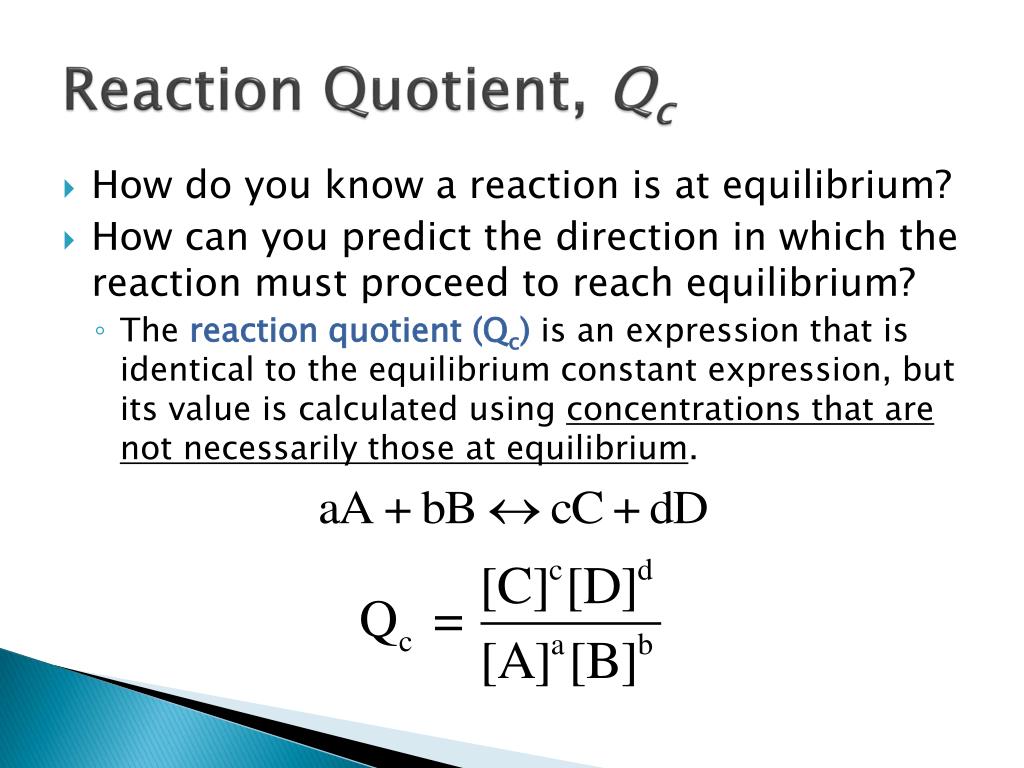
PPT - 7.4 Predicting the Direction of a Reaction PowerPoint Presentation - ID:2601616

Equilibrium Constant & Reaction Quotient
At 364 K, the equilibrium constant for the reaction: 2NO(g) ↔ N2(g) + O2(g) is Kc = 0.00307. If the initial concentration of NO is 0.153 M, what is the equilibrium concentration

ANSWERED] Predict the equilibrium concentration of O, in the
- H2 Gas Reacting with I2 Gas to Form HI Gas - Stock Image - C030/7536 - Science Photo Library

- Tênis Reebok Club C Form HI Feminino
- Zaha Hadid Architects' Latest Design Is the World's First Free-Form Exoskeleton Skyscraper

- Hi-Way 9 : Forms : Alberta, Canada Trucking Company : Logistics, Truckload, LTL, freight shipping and cargo transportation for Edmonton, Calgary, Alberta and Western Canada

- Free Hawaii Power of Attorney Forms

- Personalised Memorial Plaque - Laser Engraved, Various Designs, Add a Photo Option, Suitable for Indoors and Outdoors, Weatherproof, Gold/Silver/Copper/White (Graphic) : : Patio, Lawn & Garden

- Kid's Comfy Cami Top Solid Color Camisole Breathable - Temu

- SKIMS Swim Long Sleeve One Piece Almond - SS22 - US

- Zivame Women Underwired T-Shirt Bra, Color: Toasted Almond, Size: 34B price in UAE, UAE

- Luxury Passport Wallet - Best Travel Gift for Friend - Full Grain
