The compressibility factor for a real gas at high pressure is (a) 1+RT/pb (b) 1 (c) 1+pb/RT (d) 1-pb/RT - Sarthaks eConnect
By A Mystery Man Writer
The compressibility factor for a real gas at high pressure is (a) 1+RT/pb (b) 1 (c) 1+pb/RT (d) 1-pb/RT

The compressibility factor a real gas high pressure is: Pb RT Pb RT

gas laws - Graph of compressibility factor vs pressure when real

Real Gases Introductory Chemistry

The compressibility factor of a gas is defined as Z=PV/nRT. The

3.2 Real gas and compressibility factor – Introduction to

Calculate the compressibility factor for a gas, if 1 mole of it

At moderate pressure, the compressibility factor a particular gas

What is compressibility factor? What is its value for ideal gas

States Of Matter Notes: Class 11, JEE, NEET, AIIMS

014- 1-pb/RT The compressibility factor a real gas high pressure

The compressibility factor a real gas high pressure is: Pb RT Pb RT
- Compressibility Factor Z
- 2) 1:12:15 (3) 12:15: Jals (4) 2 5 The compressibility factor nitrogen 330 K and 800 atm is 1.90 and 200 atm is 1.10.A certain mass of Noccupies a volume of 1

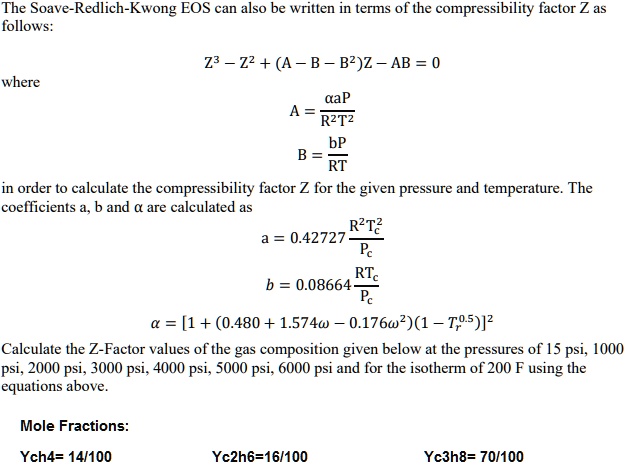
- SOLVED: The Soave-Redlich-Kwong EOS can also be written in terms of the compressibility factor Z as follows: Z^3 - Z^2 + (A - B - B^2Z - AB) = 0 where A =
- SOLVED: The compressibility factor, z, is used for predicting the behavior of non-ideal gases. How is the compressibility factor defined relative to an ideal gas? (Subscript c refers to critical value.) a)

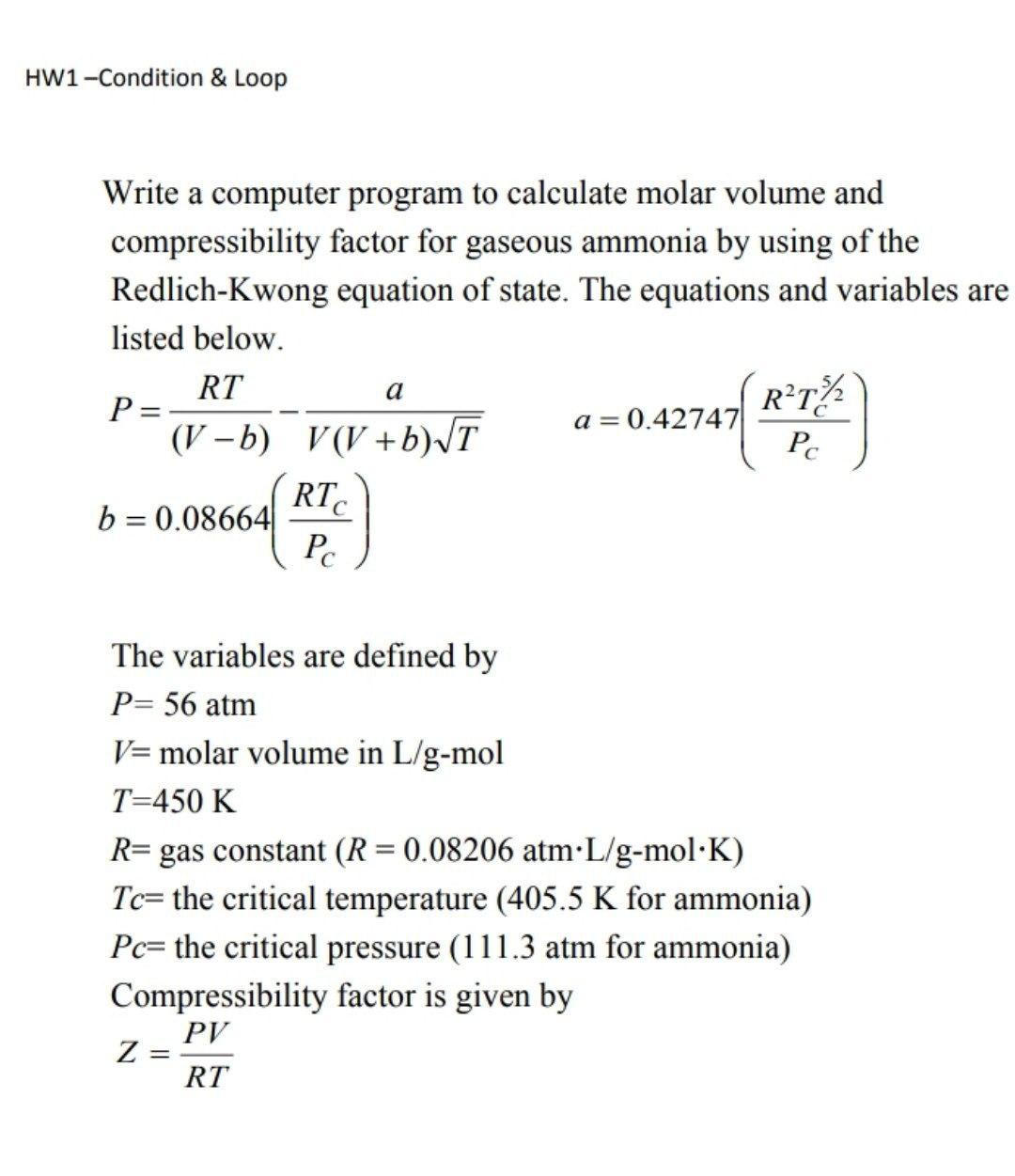
- Solved HW1-Condition & Loop Write a computer program to