UNUB At Boyle temperature, the value of compressi factor Z has a

By A Mystery Man Writer
Click here:point_up_2:to get an answer to your question :writing_hand:unubat boyle temperature the value of compressifactor z has a value of one over a
Click here👆to get an answer to your question ✍️ UNUB At Boyle temperature- the value of compressi factor Z has a value of one over a wide range of pressure- This is due to the fact that in the van der Waals equation -1- The constant a is negligible and not b -2- The constant b is negligible and not a -3- Both the constant a and b are negligible -4- Attraction balances repulsion
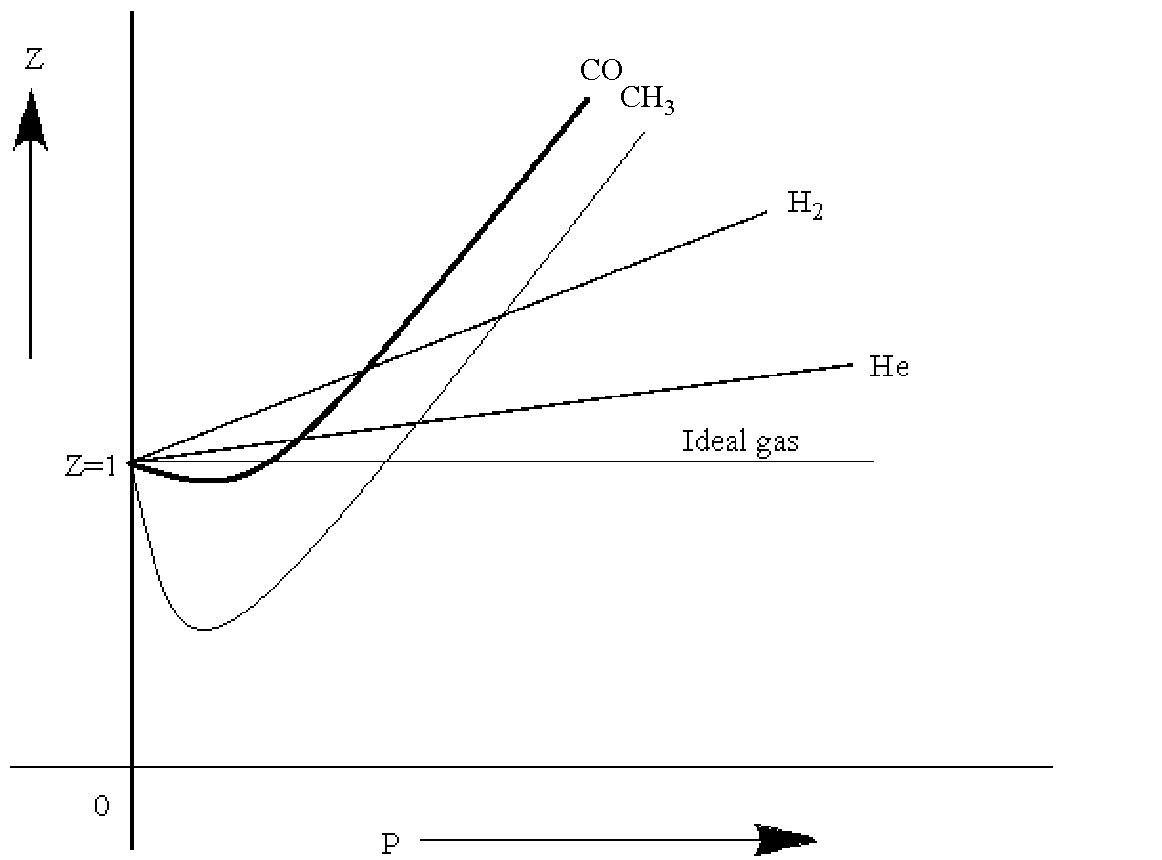
Boyle's temperature or Boyle point is the temperature at which a real gas starts behaving like an ideal gas over a particular range of pressure. A graph is plotted between the compressibility

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

Chemistry_1 - Flipbook by NOWFIYA N

COMPRESSIBILITY factor Z, Using P and v in 3 Minutes!
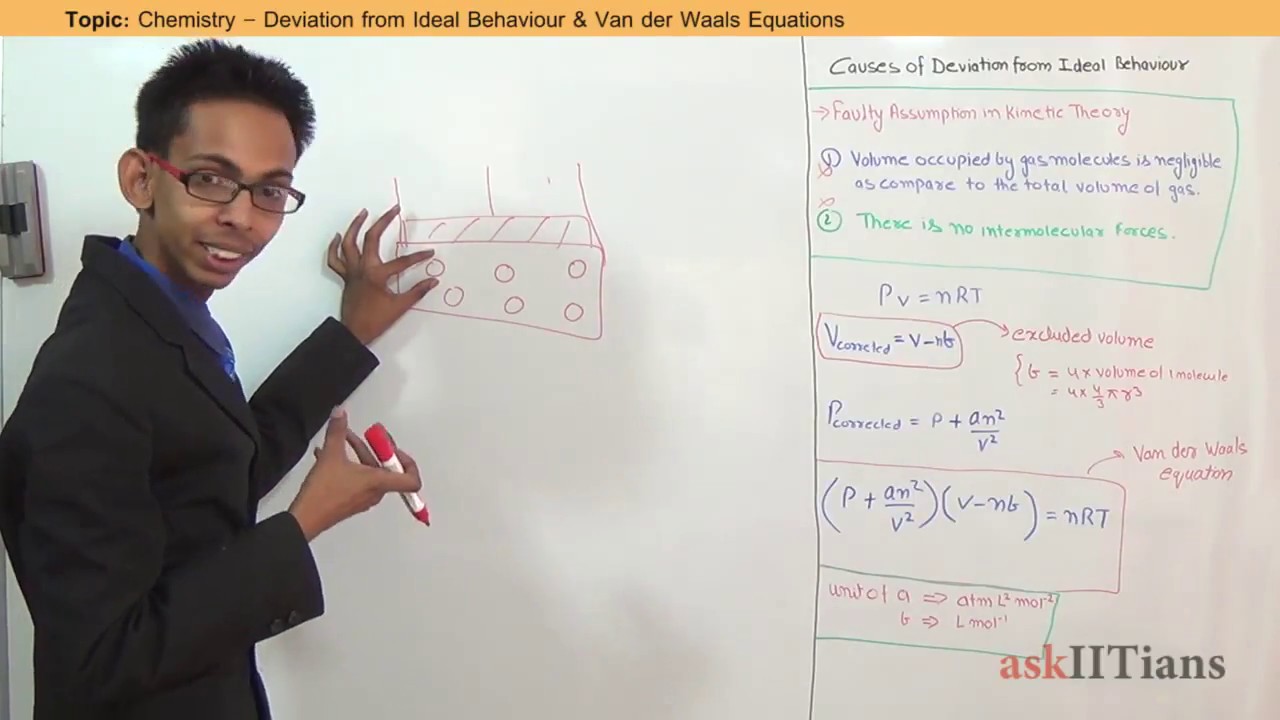
Deviation From Ideal Gas Behavior - Study Material for IIT JEE

Determine Compressibility of Gases

Thermodynamics - 3-7 Ideal Gas Equation with compressibility factor example 1
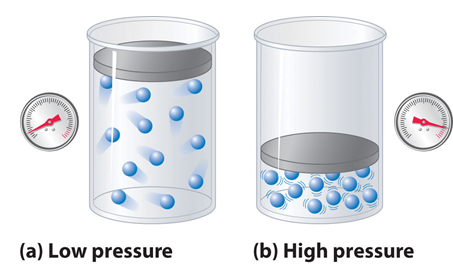
Deviation From Ideal Gas Behavior - Study Material for IIT JEE

What is the compressibility factor (Z) for 0.02 mole of a van der Waals's gas at pressure of 0

SOLVED: The compression factor Z reveals information about intermolecular interactions in real gas. Briefly describe how the values of compression factor Z, varies with pressure (i.e. at low moderate and high pressure).

qph.cf2.quoracdn.net/main-thumb-30453142-200-bmcwo
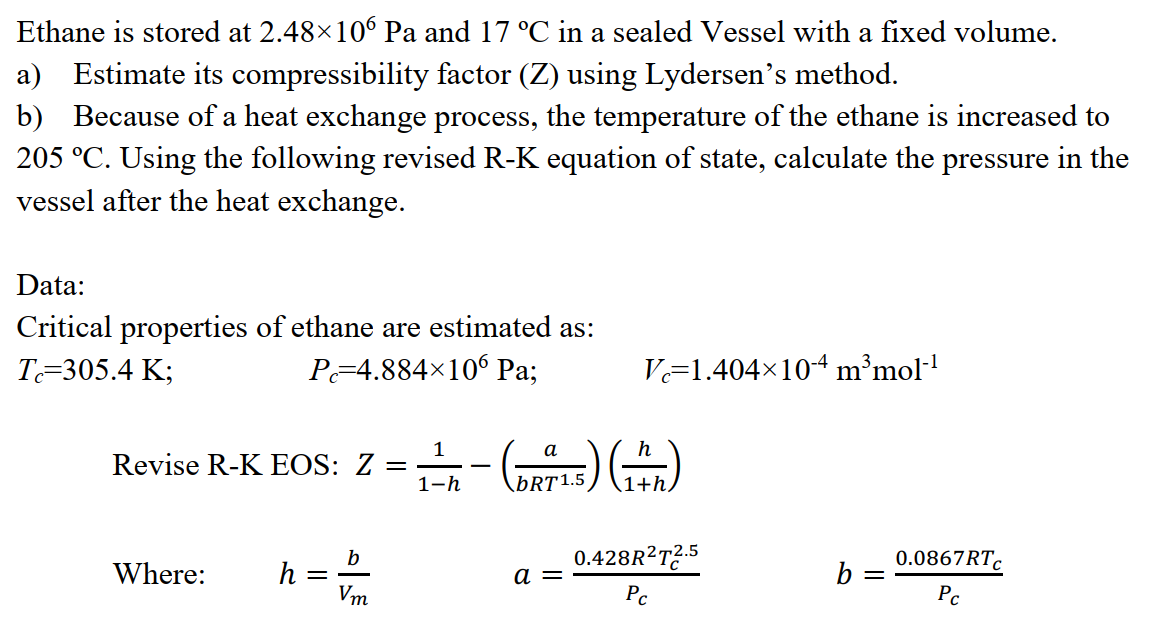
Solved Ethane is stored at 2.48x10° Pa and 17 °C in a sealed
- Solved 2. By definition, the compression factor of an ideal

- What is the compression ratio, and how is it calculated? - Quora
- Show that the van der Waals equation leads to values of Z <

- Answer in General Chemistry for Carl #275533
- SOLVED: Derive an expression for the compression factor of a gas that obeys the equation of state p(V - nb) = nRT, where b and R are constants. If the pressure and

- BALLPOINT PEN BLACK BOX OF 50 BIRO

- Buy Bong ButiQ Saree For Women Color Block Bollywood Handloom Pure Cotton Saree (Magenta, Gold) Online at Best Prices in India - JioMart.
)
- Traditional Design Maggam Work Hip Belt #44618

- Women's Lingerie, Sleep & Lounge Bra Set Mini Solid Black M

- Wholesale Cheap Soft Leggings Custom Mature Women High Waist Yoga Pants - China Yoga Wear and Sexy Yoga Bra price
