What is the value of compressibility factor in terms of vander waal cons†an t at different conditions of pressure and volume?Why is Z>1 for H2 and He gas
By A Mystery Man Writer
What is the value of compressibility factor in terms of vander waal cons†an t at different conditions of pressure and volume?Why is Z>1 for H2 and He gas
What is the value of compressibility factor in terms of vander waal cons-an t at different conditions of pressure and volume-Why is Z-1 for H2 and He gas
Gas Laws - Overview - Chemistry LibreTexts

Thermodynamics - 3-7 Ideal Gas Equation with compressibility factor example 1

Thermodynamics - 3-7 Ideal Gas Equation with compressibility factor example 1
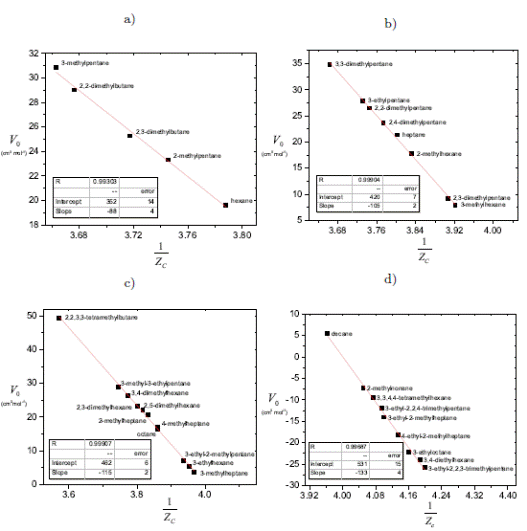
Critical Constants Correlation from van der Waals Equation

Compressibility factor (Z) for a van der Waals real gas at critical point is

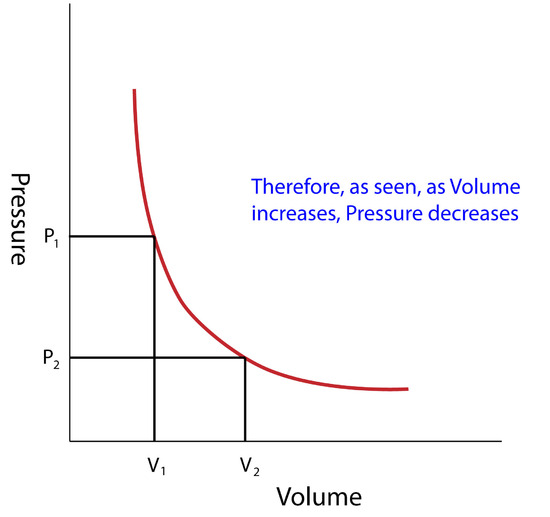
state of matter gases and liquids
If Z is a compressibility factor, van der Waals' equation at low pressure can be written as - Sarthaks eConnect
Gas Laws – First Year General Chemistry

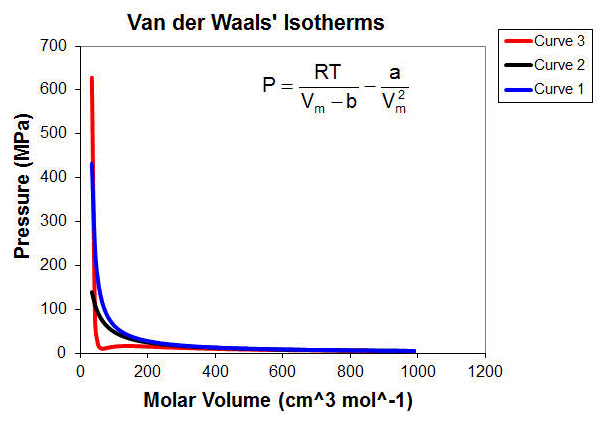
Solved Task 3 For the van der Waals gas, the compression

Solved We begin by showing that the compressibility factor

Compressibility factor - Wikipedia

Fluids, Free Full-Text
- Compressibility factor (Z) for a van der Waals real gas at critical point is

- Compressibility Factor Z
- Compressibility Factor Calculator - File Exchange - MATLAB Central

- 2) 1:12:15 (3) 12:15: Jals (4) 2 5 The compressibility factor nitrogen 330 K and 800 atm is 1.90 and 200 atm is 1.10.A certain mass of Noccupies a volume of 1

- Real gasses For an ideal gas, the compressibility factor Z = PV

- Kliou Mesh Patchwork Print Leggings Women Hipster Y2k Stitching

- KOSIWEGO Little Girls Socks, Big Toddler Cute Kuwait

- Maui Rippers, The impact of the wildfire in Lahaina has been truly overwhelming. We are utterly devastated for those who have lost their homes, busines
- Hanes Women's Original Stretch Vintage Boxer Brief - 3 Pack

- Shop Sustainable Hemp Sports Bras – Beyond Hemp Wear


